An Integrated Bioinformatics Study of a Novel Niclosamide Derivative, NSC765689, a Potential GSK3β/ β-Catenin/ STAT3/ CD44 Suppressor with Anti-Glioblastoma Properties
- PMID: 33671112
- PMCID: PMC7957701
- DOI: 10.3390/ijms22052464
An Integrated Bioinformatics Study of a Novel Niclosamide Derivative, NSC765689, a Potential GSK3β/ β-Catenin/ STAT3/ CD44 Suppressor with Anti-Glioblastoma Properties
Abstract
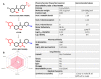
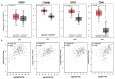
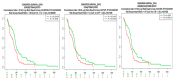
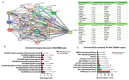
Despite management efforts with standard surgery, radiation, and chemotherapy, glioblastoma multiform (GBM) remains resistant to treatment, which leads to tumor recurrence due to glioma stem cells (GSCs) and therapy resistance. In this study, we used random computer-based prediction and target identification to assess activities of our newly synthesized niclosamide-derived compound, NSC765689, to target GBM oncogenic signaling. Using target prediction analyses, we identified glycogen synthase kinase 3β (GSK3β), β-Catenin, signal transducer and activator of transcription 3 (STAT3), and cluster of differentiation 44 (CD44) as potential druggable candidates of NSC765689. The above-mentioned signaling pathways were also predicted to be overexpressed in GBM tumor samples compared to adjacent normal samples. In addition, using bioinformatics tools, we also identified microRNA (miR)-135b as one of the most suppressed microRNAs in GBM samples, which was reported to be upregulated through inhibition of GSK3β, and subsequently suppresses GBM tumorigenic properties and stemness. We further performed in silico molecular docking of NSC765689 with GBM oncogenes; GSK3β, β-Catenin, and STAT3, and the stem cell marker, CD44, to predict protein-ligand interactions. The results indicated that NSC765689 exhibited stronger binding affinities compared to its predecessor, LCC09, which was recently published by our laboratory, and was proven to inhibit GBM stemness and resistance. Moreover, we used available US National Cancer Institute (NCI) 60 human tumor cell lines to screen in vitro anticancer effects, including the anti-proliferative and cytotoxic activities of NSC765689 against GBM cells, and 50% cell growth inhibition (GI50) values ranged 0.23~5.13 μM. In summary, using computer-based predictions and target identification revealed that NSC765689 may be a potential pharmacological lead compound which can regulate GBM oncogene (GSK3β/β-Catenin/STAT3/CD44) signaling and upregulate the miR-135b tumor suppressor. Therefore, further in vitro and in vivo investigations will be performed to validate the efficacy of NSC765689 as a novel potential GBM therapeutic.
Keywords: drug resistance; glioblastoma multiforme (GBM); glioma stem cell (GSC); in silico molecular docking; miR-135b; stemness; tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
miR-135b suppresses tumorigenesis in glioblastoma stem-like cells impairing proliferation, migration and self-renewal.Oncotarget. 2015 Nov 10;6(35):37241-56. doi: 10.18632/oncotarget.5925. Oncotarget. 2015. PMID: 26437223 Free PMC article.
-
Effect of the STAT3 inhibitor STX-0119 on the proliferation of cancer stem-like cells derived from recurrent glioblastoma.Int J Oncol. 2013 Jul;43(1):219-27. doi: 10.3892/ijo.2013.1916. Epub 2013 Apr 23. Int J Oncol. 2013. PMID: 23612755
-
The interference of Notch1 target Hes1 affects cell growth, differentiation and invasiveness of glioblastoma stem cells through modulation of multiple oncogenic targets.Oncotarget. 2017 Mar 14;8(11):17873-17886. doi: 10.18632/oncotarget.15013. Oncotarget. 2017. PMID: 28157712 Free PMC article.
-
STAT3 as a Therapeutic Target for Glioblastoma.Anticancer Agents Med Chem. 2010 Sep;10(7):512-9. doi: 10.2174/187152010793498636. Anticancer Agents Med Chem. 2010. PMID: 20879983 Review.
-
Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene.BMC Cancer. 2022 Jun 11;22(1):642. doi: 10.1186/s12885-022-09682-2. BMC Cancer. 2022. PMID: 35690717 Free PMC article. Review.
Cited by
-
Exploring the impact of mitochondrial-targeting anthelmintic agents with GLUT1 inhibitor BAY-876 on breast cancer cell metabolism.BMC Cancer. 2024 Nov 16;24(1):1415. doi: 10.1186/s12885-024-13186-6. BMC Cancer. 2024. PMID: 39550554 Free PMC article.
-
Identification of key microRNAs regulating ELOVL6 and glioblastoma tumorigenesis.BBA Adv. 2023 Jan 22;3:100078. doi: 10.1016/j.bbadva.2023.100078. eCollection 2023. BBA Adv. 2023. PMID: 37082255 Free PMC article.
-
Multiomics Study of a Novel Naturally Derived Small Molecule, NSC772864, as a Potential Inhibitor of Proto-Oncogenes Regulating Cell Cycle Progression in Colorectal Cancer.Cells. 2023 Jan 16;12(2):340. doi: 10.3390/cells12020340. Cells. 2023. PMID: 36672275 Free PMC article.
-
Cf-02, a novel benzamide-linked small molecule, blunts NF-κB activation and NLRP3 inflammasome assembly and improves acute onset of accelerated and severe lupus nephritis in mice.FASEB J. 2021 Aug;35(8):e21785. doi: 10.1096/fj.202100047R. FASEB J. 2021. PMID: 34314075 Free PMC article.
-
In-Silico Evaluation of Genetic Alterations in Ovarian Carcinoma and Therapeutic Efficacy of NSC777201, as a Novel Multi-Target Agent for TTK, NEK2, and CDK1.Int J Mol Sci. 2021 May 31;22(11):5895. doi: 10.3390/ijms22115895. Int J Mol Sci. 2021. PMID: 34072728 Free PMC article.
References
-
- Serrano-Garrido O., Peris-Torres C., Redondo-Garcia S., Asenjo H.G., Plaza-Calonge M.D.C., Fernandez-Luna J.L., Rodriguez-Manzaneque J.C. ADAMTS1 Supports Endothelial Plasticity of Glioblastoma Cells with Relevance for Glioma Progression. Biomolecules. 2020;11:44. doi: 10.3390/biom11010044. - DOI - PMC - PubMed
-
- Weller M., van den Bent M., Tonn J.C., Stupp R., Preusser M., Cohen-Jonathan-Moyal E., Henriksson R., Le Rhun E., Balana C., Chinot O., et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

