Luminescent Behavior of Gels and Sols Comprised of Molecular Gelators
- PMID: 33671130
- PMCID: PMC8005951
- DOI: 10.3390/gels7010019
Luminescent Behavior of Gels and Sols Comprised of Molecular Gelators
Abstract
We present a brief review of some important conceptual and practical aspects for the design and properties of molecular luminescent gelators and their gels. Topics considered include structural and dynamic aspects of the gels, including factors important to their ability to emit radiation from electronically excited states.
Keywords: aggregation-induced emission enhancement (AIEE), quantum yield; excimer; fluorescence; gel; luminescence; molecular organic gelator (LMOG), hydrogel; phosphorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







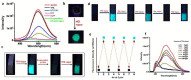


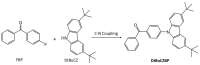
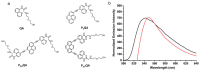
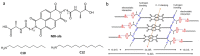

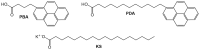
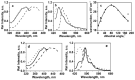
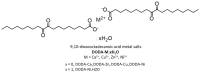
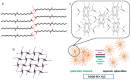
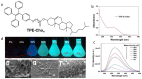

References
-
- Mondal S., Bairi P., Das S., Nandi A.K. Phase selective organogel from an imine based gelator for use in oil spill recovery. J. Mater. Chem. A. 2019;7:381–392. doi: 10.1039/C8TA09732A. - DOI
-
- Turro N.J., Ramamurthy V., Scaiano J.C. Principles of Modern Molecular Photochemistry: An Introduction. University Science Books; Sausalito, CA, USA: 2010.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

