Renal Proximal Tubule Cell Cannabinoid-1 Receptor Regulates Bone Remodeling and Mass via a Kidney-to-Bone Axis
- PMID: 33671138
- PMCID: PMC7922053
- DOI: 10.3390/cells10020414
Renal Proximal Tubule Cell Cannabinoid-1 Receptor Regulates Bone Remodeling and Mass via a Kidney-to-Bone Axis
Abstract
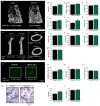
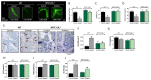
The renal proximal tubule cells (RPTCs), well-known for maintaining glucose and mineral homeostasis, play a critical role in the regulation of kidney function and bone remodeling. Deterioration in RPTC function may therefore lead to the development of diabetic kidney disease (DKD) and osteoporosis. Previously, we have shown that the cannabinoid-1 receptor (CB1R) modulates both kidney function as well as bone remodeling and mass via its direct role in RPTCs and bone cells, respectively. Here we employed genetic and pharmacological approaches that target CB1R, and found that its specific nullification in RPTCs preserves bone mass and remodeling both under normo- and hyper-glycemic conditions, and that its chronic blockade prevents the development of diabetes-induced bone loss. These protective effects of negatively targeting CB1R specifically in RPTCs were associated with its ability to modulate erythropoietin (EPO) synthesis, a hormone known to affect bone mass and remodeling. Our findings highlight a novel molecular mechanism by which CB1R in RPTCs remotely regulates skeletal homeostasis via a kidney-to-bone axis that involves EPO.
Keywords: CB1 receptor; erythropoietin; osteoporosis; type 1 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

