Non-Lethal Sequential Individual Monitoring of Viremia in Relation to DNA Vaccination in Fish-Example Using a Salmon Alphavirus DNA Vaccine in Atlantic Salmon Salmo salar
- PMID: 33671162
- PMCID: PMC7922653
- DOI: 10.3390/vaccines9020163
Non-Lethal Sequential Individual Monitoring of Viremia in Relation to DNA Vaccination in Fish-Example Using a Salmon Alphavirus DNA Vaccine in Atlantic Salmon Salmo salar
Abstract
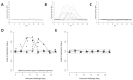
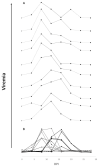
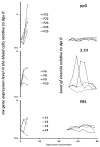
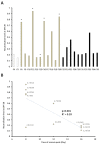
Traditionally, commercial testing for vaccine efficacy has relied on the mass infection of vaccinated and unvaccinated animals and the comparison of mortality prevalence and incidence. For some infection models where disease does not cause mortality this approach to testing vaccine efficacy is not useful. Additionally, in fish experimental studies on vaccine efficacy and immune response the norm is that several individuals are lethally sampled at sequential timepoints, and results are extrapolated to represent the kinetics of immune and disease parameters of an individual fish over the entire experimental infection period. In the present study we developed a new approach to vaccine testing for viremic viruses in fish by following the same individuals over the course of a DNA vaccination and experimental infection through repeated blood collection and analyses. Injectable DNA vaccines are particularly efficient against viral disease in fish. To date, two DNA vaccines have been authorised for use in fish farming, one in Canada against Infectious Haemorrhagic Necrotic virus and more recently one in Europe against Salmon Pancreatic Disease virus (SPDv) subtype 3. In the current study we engineered and used an experimental DNA vaccine against SPDv subtype 1. We measured viremia using a reporter cell line system and demonstrated that the viremia phase was completely extinguished following DNA vaccination. Differences in viremia infection kinetics between fish in the placebo group could be related to subsequent antibody levels in the individual fish, with higher antibody levels at terminal sampling in fish showing earlier viremia peaks. The results indicate that sequential non-lethal sampling can highlight associations between infection traits and immune responses measured at asynchronous timepoints and, can provide biological explanations for variation in data. Similar to results observed for the SPDv subtype 3 DNA vaccine, the SPDv subtype 1 DNA vaccine also induced an interferon type 1 response after vaccination and provided high protection against SPDv under laboratory conditions when fish were challenged at 7 weeks post-vaccination.
Keywords: DNA vaccine; SAV1; Viremia; individual fish monitoring; reporter assay; salmon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Pathogenesis and immune response in Atlantic salmon (Salmo salar L.) parr experimentally infected with salmon pancreas disease virus (SPDV).Fish Shellfish Immunol. 2002 Jan;12(1):77-95. doi: 10.1006/fsim.2001.0356. Fish Shellfish Immunol. 2002. PMID: 11866132
-
Effect of pancreas disease vaccines on infection levels and virus transmission in Atlantic salmon (Salmo salar) challenged with salmonid alphavirus, genotype 2.Front Immunol. 2024 Mar 7;15:1342816. doi: 10.3389/fimmu.2024.1342816. eCollection 2024. Front Immunol. 2024. PMID: 38515753 Free PMC article.
-
Isolation of salmon pancreas disease virus (SPDV) in cell culture and its ability to protect against infection by the 'wild-type' agent.Fish Shellfish Immunol. 2001 Aug;11(6):505-22. doi: 10.1006/fsim.2000.0330. Fish Shellfish Immunol. 2001. PMID: 11556480
-
Molecular biological assessment methods and understanding the course of the HIV infection.APMIS Suppl. 2003;(114):1-37. APMIS Suppl. 2003. PMID: 14626050 Review.
-
Immunization with viral antigens: infectious pancreatic necrosis.Dev Biol Stand. 1997;90:191-9. Dev Biol Stand. 1997. PMID: 9270848 Review.
Cited by
-
Unlocking Immune Signatures: Surrogate Markers for Assessing VHSV Vaccine Efficacy in Olive Flounder (Paralichthys olivaceus).Animals (Basel). 2025 Jun 11;15(12):1728. doi: 10.3390/ani15121728. Animals (Basel). 2025. PMID: 40564280 Free PMC article.
-
Editorial of Special Issue "The 2nd Edition: Vaccines for Aquaculture".Vaccines (Basel). 2022 Aug 3;10(8):1242. doi: 10.3390/vaccines10081242. Vaccines (Basel). 2022. PMID: 36016130 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

