Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle
- PMID: 33671192
- PMCID: PMC7922199
- DOI: 10.3390/biology10020158
Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle
Abstract
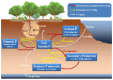
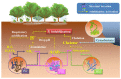
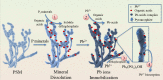
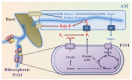
Phosphorus (P) is a vital element in biological molecules, and one of the main limiting elements for biomass production as plant-available P represents only a small fraction of total soil P. Increasing global food demand and modern agricultural consumption of P fertilizers could lead to excessive inputs of inorganic P in intensively managed croplands, consequently rising P losses and ongoing eutrophication of surface waters. Despite phosphate solubilizing microorganisms (PSMs) are widely accepted as eco-friendly P fertilizers for increasing agricultural productivity, a comprehensive and deeper understanding of the role of PSMs in P geochemical processes for managing P deficiency has received inadequate attention. In this review, we summarize the basic P forms and their geochemical and biological cycles in soil systems, how PSMs mediate soil P biogeochemical cycles, and the metabolic and enzymatic mechanisms behind these processes. We also highlight the important roles of PSMs in the biogeochemical P cycle and provide perspectives on several environmental issues to prioritize in future PSM applications.
Keywords: P biogeochemical cycle; P forms; phosphate solubilizing microorganisms; soil P.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Billah M., Khan M., Bano A., Hassan T.U., Munir A., Gurmani A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019;36:904–916. doi: 10.1080/01490451.2019.1654043. - DOI
-
- Tate K.R. The biological transformation of P in soil. Plant Soil. 1984;76:245–256. doi: 10.1007/BF02205584. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

