Tumor Environment-Responsive Hyaluronan Conjugated Zinc Protoporphyrin for Targeted Anticancer Photodynamic Therapy
- PMID: 33671291
- PMCID: PMC7922489
- DOI: 10.3390/jpm11020136
Tumor Environment-Responsive Hyaluronan Conjugated Zinc Protoporphyrin for Targeted Anticancer Photodynamic Therapy
Abstract
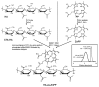
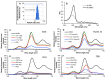
Targeted tumor accumulation, tumor environment responsive drug release, and effective internalization are critical issues being considered in developing anticancer nanomedicine. In this context, we synthesized a tumor environment-responsive nanoprobe for anticancer photodynamic therapy (PDT) that is a hyaluronan conjugated zinc protoporphyrin via an ester bond (HA-es-ZnPP), and we examined its anticancer PDT effect both in vitro and in vivo. HA-es-ZnPP exhibits high water-solubility and forms micelles of ~40 nm in aqueous solutions. HA-es-ZnPP shows fluorescence quenching without apparent 1O2 generation under light irradiation because of micelle formation. However, 1O2 was extensively generated when the micelle is disrupted, and ZnPP is released. Compared to native ZnPP, HA-es-ZnPP showed lower but comparable intracellular uptake and cytotoxicity in cultured mouse C26 colon cancer cells; more importantly, light irradiation resulted in 10-time increased cytotoxicity, which is the PDT effect. In a mouse sarcoma S180 solid tumor model, HA-es-ZnPP as polymeric micelles exhibited a prolonged systemic circulation time and the consequent tumor-selective accumulation based on the enhanced permeability and retention (EPR) effect was evidenced. Consequently, a remarkable anticancer PDT effect was achieved using HA-es-ZnPP and a xenon light source, without apparent side effects. These findings suggest the potential of HA-es-ZnPP as a candidate anticancer nanomedicine for PDT.
Keywords: EPR effect; hyaluronan; photodynamic; tumor targeting; zinc protoporphyrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

