Potential Treatment of Lysosomal Storage Disease through Modulation of the Mitochondrial-Lysosomal Axis
- PMID: 33671306
- PMCID: PMC7921977
- DOI: 10.3390/cells10020420
Potential Treatment of Lysosomal Storage Disease through Modulation of the Mitochondrial-Lysosomal Axis
Abstract
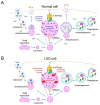
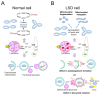
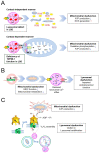
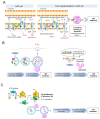
Lysosomal storage disease (LSD) is an inherited metabolic disorder caused by enzyme deficiency in lysosomes. Some treatments for LSD can slow progression, but there are no effective treatments to restore the pathological phenotype to normal levels. Lysosomes and mitochondria interact with each other, and this crosstalk plays a role in the maintenance of cellular homeostasis. Deficiency of lysosome enzymes in LSD impairs the turnover of mitochondrial defects, leading to deterioration of the mitochondrial respiratory chain (MRC). Cells with MRC impairment are associated with reduced lysosomal calcium homeostasis, resulting in impaired autophagic and endolysosomal function. This malicious feedback loop between lysosomes and mitochondria exacerbates LSD. In this review, we assess the interactions between mitochondria and lysosomes and propose the mitochondrial-lysosomal axis as a research target to treat LSD. The importance of the mitochondrial-lysosomal axis has been systematically characterized in several studies, suggesting that proper regulation of this axis represents an important investigative guide for the development of therapeutics for LSD. Therefore, studying the mitochondrial-lysosomal axis will not only add knowledge of the essential physiological processes of LSD, but also provide new strategies for treatment of LSD.
Keywords: lysosomal storage disease; lysosome; mitochondria; mitochondrial–lysosomal axis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Chapel A., Kieffer-Jaquinod S., Sagné C., Verdon Q., Ivaldi C., Mellal M., Thirion J., Jadot M., Bruley C., Garin J., et al. An extended proteome map of the lysosomal membrane reveals novel potential transporters. Mol. Cell. Proteom. MCP. 2013;12:1572–1588. doi: 10.1074/mcp.M112.021980. - DOI - PMC - PubMed
-
- Di Fruscio G., Schulz A., De Cegli R., Savarese M., Mutarelli M., Parenti G., Banfi S., Braulke T., Nigro V., Ballabio A. Lysoplex: An efficient toolkit to detect DNA sequence variations in the autophagy-lysosomal pathway. Autophagy. 2015;11:928–938. doi: 10.1080/15548627.2015.1043077. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

