Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering
- PMID: 33671329
- PMCID: PMC7923188
- DOI: 10.3390/polym13040599
Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering
Abstract
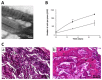
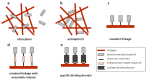
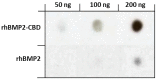
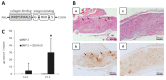
Collagen type I is the main organic constituent of the bone extracellular matrix and has been used for decades as scaffolding material in bone tissue engineering approaches when autografts are not feasible. Polymeric collagen can be easily isolated from various animal sources and can be processed in a great number of ways to manufacture biomaterials in the form of sponges, particles, or hydrogels, among others, for different applications. Despite its great biocompatibility and osteoconductivity, collagen type I also has some drawbacks, such as its high biodegradability, low mechanical strength, and lack of osteoinductive activity. Therefore, many attempts have been made to improve the collagen type I-based implants for bone tissue engineering. This review aims to summarize the current status of collagen type I as a biomaterial for bone tissue engineering, as well as to highlight some of the main efforts that have been made recently towards designing and producing collagen implants to improve bone regeneration.
Keywords: bone tissue engineering; collagen; growth factors; peptides; scaffolds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

