A GMMA-CPS-Based Vaccine for Non-Typhoidal Salmonella
- PMID: 33671372
- PMCID: PMC7922415
- DOI: 10.3390/vaccines9020165
A GMMA-CPS-Based Vaccine for Non-Typhoidal Salmonella
Abstract
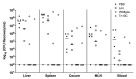
Non-typhoidal Salmonella are a major cause of gastroenteritis worldwide, as well as causing bloodstream infections in sub-Saharan Africa with a high fatality rate. No vaccine is currently available for human use. Current vaccine development strategies are focused on capsular polysaccharides (CPS) present on the surface of non-typhoidal Salmonella. This study aimed to boost the amount of CPS purified from S. Typhimurium for immunization trials. Random mutagenesis with Tn10 transposon increased the production of CPS colanic acid, by 10-fold compared to wildtype. Immunization with colanic acid or colanic acid conjugated to truncated glycoprotein D or inactivated diphtheria toxin did not induce a protective immune response in mice. However, immunization with Generalized Modules for Membrane Antigens (GMMAs) isolated from colanic acid overproducing isolates reduced Salmonella colonization in mice. Our results support the development of a GMMA-CPS-based vaccine against non-typhoidal Salmonella.
Keywords: GMMAs; Salmonella; capsular polysaccharide; colanic acid; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
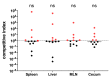

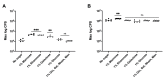
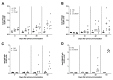
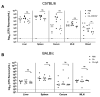

References
-
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. - DOI - PMC - PubMed
-
- Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’Brien S.J., Jones T.F., Fazil A., Hoekstra R.M., for the International Collaboration on Enteric Disease “Burden of Illness” Studies The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. - DOI - PubMed
-
- Eng S.-K., Pusparajah P., Mutalib N.-S.A., Ser H.-L., Chan K.-G., Lee L.-H. Salmonella: A Review on Pathogenesis, Epidemiology and Antibiotic Resistance. Front. Life Sci. 2015;8:284–293. doi: 10.1080/21553769.2015.1051243. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

