Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus
- PMID: 33671399
- PMCID: PMC7922993
- DOI: 10.3390/vaccines9020167
Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus
Abstract
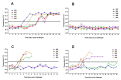
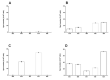
Live attenuated C-strain classical swine fever vaccines provide early onset protection. These vaccines confer effective protection against the disease at 5-7 days post-vaccination. It was previously reported that intramuscular administration of the Porvac® vaccine protects against highly virulent classical swine fever virus (CSFV) "Margarita" strain as early as seven days post-vaccination. In order to identify how rapidly protection against CSFV is conferred after a single dose of the Porvac® subunit vaccine E2-CD154, 15 swine, vaccinated with a single dose of Porvac®, were challenged intranasally at five, three, and one day post-vaccination with 2 × 103 LD50 of the highly pathogenic Cuban "Margarita" strain of the classical swine fever virus. Another five animals were the negative control of the experiment. The results provided clinical and virological data confirming protection at five days post-vaccination. Classical swine fever (CSF)-specific IFNγ T cell responses were detected in vaccinated animals but not detected in unvaccinated control animals. These results provided the first data that a subunit protein vaccine demonstrates clinical and viral protection at five days post-vaccination, as modified live vaccines.
Keywords: Porvac® subunit vaccine E2-CD154; T cell IFNγ responses; classical swine fever virus; early protection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

