Activation of Apoptosis in a βB1-CTGF Transgenic Mouse Model
- PMID: 33671472
- PMCID: PMC7922353
- DOI: 10.3390/ijms22041997
Activation of Apoptosis in a βB1-CTGF Transgenic Mouse Model
Abstract
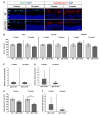
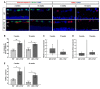
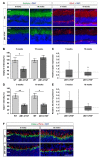
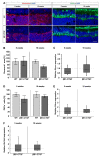
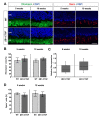
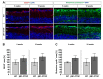
To reveal the pathomechanisms of glaucoma, a common cause of blindness, suitable animal models are needed. As previously shown, retinal ganglion cell and optic nerve degeneration occur in βB1-CTGF mice. Here, we aimed to determine possible apoptotic mechanisms and degeneration of different retinal cells. Hence, retinae were processed for immunohistology (n = 5-9/group) and quantitative real-time PCR analysis (n = 5-7/group) in 5- and 10-week-old βB1-CTGF and wildtype controls. We noted significantly more cleaved caspase 3+ cells in βB1-CTGF retinae at 5 (p = 0.005) and 10 weeks (p = 0.02), and a significant upregulation of Casp3 and Bax/Bcl2 mRNA levels (p < 0.05). Furthermore, more terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL+) cells were detected in transgenic mice at 5 (p = 0.03) and 10 weeks (p = 0.02). Neurofilament H staining (p = 0.01) as well as Nefh (p = 0.02) and Tubb3 (p = 0.009) mRNA levels were significantly decreased at 10 weeks. GABAergic synapse intensity was lower at 5 weeks, while no alterations were noted at 10 weeks. The glutamatergic synapse intensity was decreased at 5 (p = 0.007) and 10 weeks (p = 0.01). No changes were observed for bipolar cells, photoreceptors, and macroglia. We conclude that apoptotic processes and synapse loss precede neuronal death in this model. This slow progression rate makes the βB1-CTGF mice a suitable model to study primary open-angle glaucoma.
Keywords: Bax/Bcl2; apoptosis; caspase 3; neurofilament H; primary open-angle glaucoma; synapse; βB1-CTGF.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Global Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. - DOI - PubMed
-
- Kerrigan-Baumrind L.A., Quigley H.A., Pease M.E., Kerrigan D.F., Mitchell R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest. Ophthalmol. Vis. Sci. 2000;41:741–748. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

