Influence of Genistein on Hepatic Lipid Metabolism in an In Vitro Model of Hepatic Steatosis
- PMID: 33671486
- PMCID: PMC7926972
- DOI: 10.3390/molecules26041156
Influence of Genistein on Hepatic Lipid Metabolism in an In Vitro Model of Hepatic Steatosis
Abstract
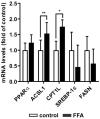
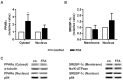
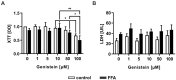
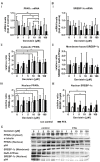
Nonalcoholic fatty liver disease (NAFLD) is among the leading causes of end-stage liver disease. The impaired hepatic lipid metabolism in NAFLD is exhibited by dysregulated PPARα and SREBP-1c signaling pathways, which are central transcription factors associated with lipid degradation and de novo lipogenesis. Despite the growing prevalence of this disease, current pharmacological treatment options are unsatisfactory. Genistein, a soy isoflavone, has beneficial effects on lipid metabolism and may be a candidate for NAFLD treatment. In an in vitro model of hepatic steatosis, primary human hepatocytes (PHHs) were incubated with free fatty acids (FFAs) and different doses of genistein. Lipid accumulation and the cytotoxic effects of FFAs and genistein treatment were evaluated by colorimetric and enzymatic assays. Changes in lipid homeostasis were examined by RT-qPCR and Western blot analyses. PPARα protein expression was induced in steatotic PHHs, accompanied by an increase in CPT1L and ACSL1 mRNA. Genistein treatment increased PPARα protein expression only in control PHHs, while CPTL1 and ACSL1 were unchanged and PPARα mRNA was reduced. In steatotic PHHs, genistein reversed the increase in activated SREBP-1c protein. The model realistically reflected the molecular changes in hepatic steatosis. Genistein suppressed the activation of SREBP-1c in steatotic hepatocytes, but the genistein-mediated effects on PPARα were abolished by high hepatic lipid levels.
Keywords: Genistein; NAFLD; NASH; PPARα; SREBP-1c; liver; primary human hepatocytes; steatosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Genistein has beneficial effects on hepatic steatosis in high fat-high sucrose diet-treated rats.Biomed Pharmacother. 2017 Jul;91:964-969. doi: 10.1016/j.biopha.2017.04.130. Epub 2017 May 13. Biomed Pharmacother. 2017. PMID: 28514835
-
Oxymatrine attenuates hepatic steatosis in non-alcoholic fatty liver disease rats fed with high fructose diet through inhibition of sterol regulatory element binding transcription factor 1 (Srebf1) and activation of peroxisome proliferator activated receptor alpha (Pparα).Eur J Pharmacol. 2013 Aug 15;714(1-3):89-95. doi: 10.1016/j.ejphar.2013.06.013. Epub 2013 Jun 18. Eur J Pharmacol. 2013. PMID: 23791610
-
Free Fatty Acids Increase Intracellular Lipid Accumulation and Oxidative Stress by Modulating PPARα and SREBP-1c in L-02 Cells.Lipids. 2016 Jul;51(7):797-805. doi: 10.1007/s11745-016-4160-y. Epub 2016 Jun 7. Lipids. 2016. PMID: 27270405
-
Effective Food Ingredients for Fatty Liver: Soy Protein β-Conglycinin and Fish Oil.Int J Mol Sci. 2018 Dec 18;19(12):4107. doi: 10.3390/ijms19124107. Int J Mol Sci. 2018. PMID: 30567368 Free PMC article. Review.
-
Possible molecular mechanisms soy-mediated in preventing and treating nonalcoholic fatty liver disease.Nutr Hosp. 2012 Jul-Aug;27(4):991-8. doi: 10.3305/nh.2012.27.4.5833. Nutr Hosp. 2012. PMID: 23165534 Review.
Cited by
-
Sex difference in liver diseases: How preclinical models help to dissect the sex-related mechanisms sustaining NAFLD and hepatocellular carcinoma.iScience. 2023 Oct 30;26(12):108363. doi: 10.1016/j.isci.2023.108363. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034347 Free PMC article. Review.
-
Protective effect of phytoestrogens on nonalcoholic fatty liver disease in postmenopausal women.Front Pharmacol. 2023 Aug 30;14:1237845. doi: 10.3389/fphar.2023.1237845. eCollection 2023. Front Pharmacol. 2023. PMID: 37719855 Free PMC article. Review.
-
Cell Homeostasis or Cell Death-The Balancing Act Between Autophagy and Apoptosis Caused by Steatosis-Induced Endoplasmic Reticulum (ER) Stress.Cells. 2025 Mar 18;14(6):449. doi: 10.3390/cells14060449. Cells. 2025. PMID: 40136698 Free PMC article.
-
Targeting lipid droplets and lipid droplet-associated proteins: a new perspective on natural compounds against metabolic diseases.Chin Med. 2024 Sep 4;19(1):120. doi: 10.1186/s13020-024-00988-w. Chin Med. 2024. PMID: 39232826 Free PMC article. Review.
-
Primary-like Human Hepatocytes Genetically Engineered to Obtain Proliferation Competence as a Capable Application for Energy Metabolism Experiments in In Vitro Oncologic Liver Models.Biology (Basel). 2022 Aug 9;11(8):1195. doi: 10.3390/biology11081195. Biology (Basel). 2022. PMID: 36009822 Free PMC article.
References
-
- Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M., Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

