Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells
- PMID: 33671498
- PMCID: PMC7926465
- DOI: 10.3390/diagnostics11020367
Wnt/β-Catenin Signaling Regulates CXCR4 Expression and [68Ga] Pentixafor Internalization in Neuroendocrine Tumor Cells
Abstract
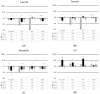
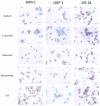
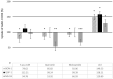
Loss of Somatostatin Receptor 2 (SSTR2) expression and rising CXC Chemokine Receptor Type 4 (CXCR4) expression are associated with dedifferentiation in neuroendocrine tumors (NET). In NET, CXCR4 expression is associated with enhanced metastatic and invasive potential and worse prognosis but might be a theragnostic target. Likewise, activation of Wnt/β-catenin signaling may promote a more aggressive phenotype in NET. We hypothesized an interaction of the Wnt/β-catenin pathway with CXCR4 expression and function in NET. The NET cell lines BON-1, QGP-1, and MS-18 were exposed to Wnt inhibitors (5-aza-CdR, quercetin, and niclosamide) or the Wnt activator LiCl. The expressions of Wnt pathway genes and of CXCR4 were studied by qRT-PCR, Western blot, and immunohistochemistry. The effects of Wnt modulators on uptake of the CXCR4 ligand [68Ga] Pentixafor were measured. The Wnt activator LiCl induced upregulation of CXCR4 and Wnt target gene expression. Treatment with the Wnt inhibitors had opposite effects. LiCl significantly increased [68Ga] Pentixafor uptake, while treatment with Wnt inhibitors decreased radiopeptide uptake. Wnt pathway modulation influences CXCR4 expression and function in NET cell lines. Wnt modulation might be a tool to enhance the efficacy of CXCR4-directed therapies in NET or to inhibit CXCR4-dependent proliferative signaling. The underlying mechanisms for the interaction of the Wnt pathway with CXCR4 expression and function have yet to be clarified.
Keywords: BON-1; CXCR4; MS-18; NET; QGP-1; Wnt; [68Ga] Pentixafor; neuroendocrine tumor; β-catenin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Kaemmerer D., Träger T., Hoffmeister M., Sipos B., Hommann M., Sänger J., Schulz S., Lupp A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget. 2015;6:27566–27579. doi: 10.18632/oncotarget.4491. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

