Lipid Droplets Are a Physiological Nucleoporin Reservoir
- PMID: 33671805
- PMCID: PMC7926788
- DOI: 10.3390/cells10020472
Lipid Droplets Are a Physiological Nucleoporin Reservoir
Abstract
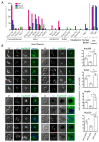
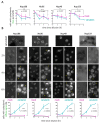
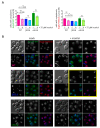
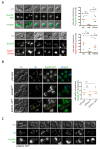
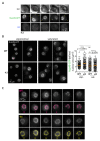
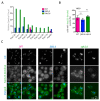
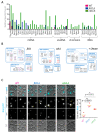
Lipid Droplets (LD) are dynamic organelles that originate in the Endoplasmic Reticulum and mostly bud off toward the cytoplasm, where they store neutral lipids for energy and protection purposes. LD also have diverse proteins on their surface, many of which are necessary for the their correct homeostasis. However, these organelles also act as reservoirs of proteins that can be made available elsewhere in the cell. In this sense, they act as sinks that titrate key regulators of many cellular processes. Among the specialized factors that reside on cytoplasmic LD are proteins destined for functions in the nucleus, but little is known about them and their impact on nuclear processes. By screening for nuclear proteins in publicly available LD proteomes, we found that they contain a subset of nucleoporins from the Nuclear Pore Complex (NPC). Exploring this, we demonstrate that LD act as a physiological reservoir, for nucleoporins, that impacts the conformation of NPCs and hence their function in nucleo-cytoplasmic transport, chromatin configuration, and genome stability. Furthermore, our in silico modeling predicts a role for LD-released fatty acids in regulating the transit of nucleoporins from LD through the cytoplasm and to nuclear pores.
Keywords: fatty acids binding; genome integrity pathways; lipid droplets; nucleoporins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Characterization of genome-nucleoporin interactions in Drosophila links chromatin insulators to the nuclear pore complex.Cell Cycle. 2010 Dec 15;9(24):4812-7. doi: 10.4161/cc.9.24.14328. Epub 2010 Dec 15. Cell Cycle. 2010. PMID: 21150273
-
mmBCFA C17iso ensures endoplasmic reticulum integrity for lipid droplet growth.J Cell Biol. 2021 Nov 1;220(11):e202102122. doi: 10.1083/jcb.202102122. Epub 2021 Oct 8. J Cell Biol. 2021. PMID: 34623380 Free PMC article.
-
Mammalian lipid droplets: structural, pathological, immunological and anti-toxicological roles.Prog Lipid Res. 2023 Jul;91:101233. doi: 10.1016/j.plipres.2023.101233. Epub 2023 May 6. Prog Lipid Res. 2023. PMID: 37156444 Review.
-
Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD.Mol Metab. 2021 Aug;50:101115. doi: 10.1016/j.molmet.2020.101115. Epub 2020 Nov 10. Mol Metab. 2021. PMID: 33186758 Free PMC article. Review.
-
Metazoan nuclear pore complexes in gene regulation and genome stability.DNA Repair (Amst). 2023 Oct;130:103565. doi: 10.1016/j.dnarep.2023.103565. Epub 2023 Sep 4. DNA Repair (Amst). 2023. PMID: 37696111 Review.
Cited by
-
Origin of gradients in lipid density and surface tension between connected lipid droplet and bilayer.Biophys J. 2021 Dec 21;120(24):5491-5503. doi: 10.1016/j.bpj.2021.11.022. Epub 2021 Nov 19. Biophys J. 2021. PMID: 34808099 Free PMC article.
-
Lack of evidence for condensin or cohesin sequestration on lipid droplets with packing defects.MicroPubl Biol. 2021 Nov 4;2021:10.17912/micropub.biology.000497. doi: 10.17912/micropub.biology.000497. eCollection 2021. MicroPubl Biol. 2021. PMID: 34746685 Free PMC article.
-
Uip4p modulates nuclear pore complex function in Saccharomyces cerevisiae.Nucleus. 2022 Dec;13(1):79-93. doi: 10.1080/19491034.2022.2034286. Nucleus. 2022. PMID: 35171083 Free PMC article.
-
New Activities of the Nuclear Pore Complexes.Cells. 2021 Aug 18;10(8):2123. doi: 10.3390/cells10082123. Cells. 2021. PMID: 34440892 Free PMC article.
-
Distinct features of two lipid droplets types in cell nuclei from patients with liver diseases.Sci Rep. 2023 Apr 26;13(1):6851. doi: 10.1038/s41598-023-33977-4. Sci Rep. 2023. PMID: 37100813 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

