Identification of Three Small Molecules That Can Selectively Influence Cellular Manganese Levels in a Mouse Striatal Cell Model
- PMID: 33671818
- PMCID: PMC7931103
- DOI: 10.3390/molecules26041175
Identification of Three Small Molecules That Can Selectively Influence Cellular Manganese Levels in a Mouse Striatal Cell Model
Abstract
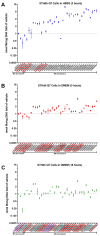
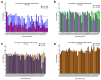
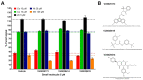
Manganese (Mn) is a biologically essential metal, critical as a cofactor for numerous enzymes such a glutamine synthetase and kinases such as ataxia-telangiectasia mutated (ATM). Similar to other essential metals such as iron and zinc, proper levels of Mn need to be achieved while simultaneously being careful to avoid excess levels of Mn that can be neurotoxic. A lifetime of occupational exposure to Mn can often lead to a Parkinsonian condition, also known as "manganism", characterized by impaired gait, muscle spasms, and tremors. Despite the importance of its regulation, the mechanisms underlying the transport and homeostasis of Mn are poorly understood. Rather than taking a protein or gene-targeted approach, our lab recently took a high-throughput-screening approach to identify 41 small molecules that could significantly increase or decrease intracellular Mn in a neuronal cell model. Here, we report characterization of these small molecules, which we refer to as the "Mn toolbox". We adapted a Fura-2-based assay for measuring Mn concentration and for measuring relative concentrations of other divalent metals: nickel, copper, cobalt, and zinc. Of these 41 small molecules, we report here the identification of three that selectively influence cellular Mn but do not influence the other divalent metals tested. The patterns of activity across divalent metals and the discovery of Mn-selective small molecules has potential pharmacological and scientific utility.
Keywords: divalent metals; manganese; metal homeostasis; metal transport; neurotoxicity; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cellular manganese content is developmentally regulated in human dopaminergic neurons.Sci Rep. 2014 Oct 28;4:6801. doi: 10.1038/srep06801. Sci Rep. 2014. PMID: 25348053 Free PMC article.
-
Phosphatidylinositol 3 kinase (PI3K) modulates manganese homeostasis and manganese-induced cell signaling in a murine striatal cell line.Neurotoxicology. 2018 Jan;64:185-194. doi: 10.1016/j.neuro.2017.07.026. Epub 2017 Aug 2. Neurotoxicology. 2018. PMID: 28780388 Free PMC article.
-
Novel high-throughput assay to assess cellular manganese levels in a striatal cell line model of Huntington's disease confirms a deficit in manganese accumulation.Neurotoxicology. 2011 Oct;32(5):630-9. doi: 10.1016/j.neuro.2011.01.002. Epub 2011 Jan 14. Neurotoxicology. 2011. PMID: 21238486 Free PMC article.
-
Manganese and the brain.Int Rev Neurobiol. 2013;110:277-312. doi: 10.1016/B978-0-12-410502-7.00013-2. Int Rev Neurobiol. 2013. PMID: 24209443 Review.
-
Manganese homeostasis in the nervous system.J Neurochem. 2015 Aug;134(4):601-10. doi: 10.1111/jnc.13170. Epub 2015 Jun 16. J Neurochem. 2015. PMID: 25982296 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

