Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting
- PMID: 33671915
- PMCID: PMC7918979
- DOI: 10.3390/ijms22041916
Long-Term Shaping of Corticostriatal Synaptic Activity by Acute Fasting
Abstract
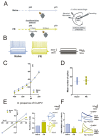
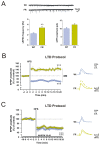
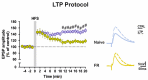
Food restriction is a robust nongenic, nonsurgical and nonpharmacologic intervention known to improve health and extend lifespan in various species. Food is considered the most essential and frequently consumed natural reward, and current observations have demonstrated homeostatic responses and neuroadaptations to sustained intermittent or chronic deprivation. Results obtained to date indicate that food deprivation affects glutamatergic synapses, favoring the insertion of GluA2-lacking α-Ammino-3-idrossi-5-Metil-4-idrossazol-Propionic Acid receptors (AMPARs) in postsynaptic membranes. Despite an increasing number of studies pointing towards specific changes in response to dietary restrictions in brain regions, such as the nucleus accumbens and hippocampus, none have investigated the long-term effects of such practice in the dorsal striatum. This basal ganglia nucleus is involved in habit formation and in eating behavior, especially that based on dopaminergic control of motivation for food in both humans and animals. Here, we explored whether we could retrieve long-term signs of changes in AMPARs subunit composition in dorsal striatal neurons of mice acutely deprived for 12 hours/day for two consecutive days by analyzing glutamatergic neurotransmission and the principal forms of dopamine and glutamate-dependent synaptic plasticity. Overall, our data show that a moderate food deprivation in experimental animals is a salient event mirrored by a series of neuroadaptations and suggest that dietary restriction may be determinant in shaping striatal synaptic plasticity in the physiological state.
Keywords: GluA1; calcium-permeable AMPA; dietary restriction; dorsolateral striatum; food deprivation; naphthyl-acetyl spermine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A High-fat, High-sugar 'Western' Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice.Neuroscience. 2018 Feb 21;372:1-15. doi: 10.1016/j.neuroscience.2017.12.036. Epub 2017 Dec 28. Neuroscience. 2018. PMID: 29289718 Free PMC article.
-
Combination of group I mGlu receptors antagonist with dopaminergic agonists strengthens the synaptic transmission at corticostriatal synapses in culture.Neuropharmacology. 2013 Mar;66:151-7. doi: 10.1016/j.neuropharm.2012.03.017. Epub 2012 Mar 23. Neuropharmacology. 2013. PMID: 22465815
-
Kainate Receptors Inhibit Glutamate Release Via Mobilization of Endocannabinoids in Striatal Direct Pathway Spiny Projection Neurons.J Neurosci. 2018 Apr 18;38(16):3901-3910. doi: 10.1523/JNEUROSCI.1788-17.2018. Epub 2018 Mar 14. J Neurosci. 2018. PMID: 29540547 Free PMC article.
-
Synaptic dysfunction in Parkinson's disease.Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
-
Integrating neurotransmission in striatal medium spiny neurons.Adv Exp Med Biol. 2012;970:407-29. doi: 10.1007/978-3-7091-0932-8_18. Adv Exp Med Biol. 2012. PMID: 22351066 Review.
Cited by
-
Dorsomedial Striatum (DMS) CB1R Signaling Promotes Pavlovian Devaluation Sensitivity in Male Long Evans Rats and Reduces DMS Inhibitory Synaptic Transmission in Both Sexes.eNeuro. 2025 Jan 29;12(1):ENEURO.0341-24.2024. doi: 10.1523/ENEURO.0341-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39746803 Free PMC article.
-
Late-Onset, Short-Term Intermittent Fasting Reverses Age-Related Changes in Calcium Buffering and Inhibitory Synaptic Transmission in Mouse Basal Forebrain Neurons.J Neurosci. 2022 Feb 9;42(6):1020-1034. doi: 10.1523/JNEUROSCI.1442-21.2021. Epub 2021 Dec 15. J Neurosci. 2022. PMID: 34911797 Free PMC article.
-
Molecular Mechanisms of Synaptic Plasticity 2.0: Dynamic Changes in Neurons Functions, Physiological and Pathological Process.Int J Mol Sci. 2023 Aug 11;24(16):12685. doi: 10.3390/ijms241612685. Int J Mol Sci. 2023. PMID: 37628862 Free PMC article.
References
-
- Chechko N., Vocke S., Habel U., Toygar T., Kuckartz L., Berthold-Losleben M., Laoutidis Z.G., Orfanos S., Wassenberg A., Karges W., et al. Effects of overnight fasting on working memory-related brain network: An fMRI study. Hum. Brain Mapp. 2015;36:839–851. doi: 10.1002/hbm.22668. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

