Early Covert Appearance of Marginal Zone B Cells in Salivary Glands of Sjögren's Syndrome-Susceptible Mice: Initiators of Subsequent Overt Clinical Disease
- PMID: 33671965
- PMCID: PMC7919007
- DOI: 10.3390/ijms22041919
Early Covert Appearance of Marginal Zone B Cells in Salivary Glands of Sjögren's Syndrome-Susceptible Mice: Initiators of Subsequent Overt Clinical Disease
Abstract
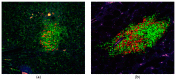
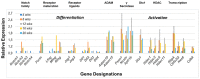
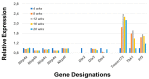
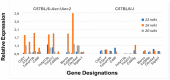
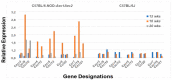
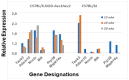
The C57BL/6.NOD-Aec1Aec2 mouse model has been extensively studied to define the underlying cellular and molecular bioprocesses critical in the onset of primary Sjögren's Syndrome (pSS), a human systemic autoimmune disease characterized clinically as the loss of lacrimal and salivary gland functions leading to dry eye and dry mouth pathologies. This mouse model, together with several gene knockout mouse models of SS, has indicated that B lymphocytes, especially marginal zone B (MZB) cells, are necessary for development and onset of clinical manifestations despite the fact that destruction of the lacrimal and salivary gland cells involves a classical T cell-mediated autoimmune response. Because migrations and functions of MZB cells are difficult to study in vivo, we have carried out ex vivo investigations that use temporal global RNA transcriptomic analyses to profile autoimmunity as it develops within the salivary glands of C57BL/6.NOD-Aec1Aec2 mice. Temporal profiles indicate the appearance of Notch2-positive cells within the salivary glands of these SS-susceptible mice concomitant with the early-phase appearance of lymphocytic foci (LF). Data presented here identify cellular bioprocesses occurring during early immune cell migrations into the salivary glands and suggest MZB cells are recruited to the exocrine glands by the upregulated Cxcl13 chemokine where they recognize complement (C')-decorated antigens via their sphingosine-1-phosphate (S1P) and B cell (BC) receptors. Based on known MZB cell behavior and mobility, we propose that MZB cells activated in the salivary glands migrate to splenic follicular zones to present antigens to follicular macrophages and dendritic cells that, in turn, promote a subsequent systemic cell-mediated and autoantibody-mediated autoimmune T cell response that targets exocrine gland cells and functions. Overall, this study uses the power of transcriptomic analyses to provide greater insight into several molecular events defining cellular bioprocesses underlying SS that can be modelled and more thoroughly studied at the cellular level.
Keywords: B cell receptor (BCR); C57BL/6.NOD-Aec1Aec2 mice; Notch2; RNA transcriptome microarrays; Sjögren’s syndrome; marginal zone B (MZB) cells; marginal zones (MZ); sphingosine-1-phosphate (S1P); sphingosine-1-phosphate receptor (S1PR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Marginal Zone B (MZB) Cells: Comparison of the Initial Identification of Immune Activity Leading to Dacryoadenitis and Sialadenitis in Experimental Sjögren's Syndrome.Int J Mol Sci. 2023 Jul 30;24(15):12209. doi: 10.3390/ijms241512209. Int J Mol Sci. 2023. PMID: 37569583 Free PMC article.
-
Upregulated Chemokine and Rho-GTPase Genes Define Immune Cell Emigration into Salivary Glands of Sjögren's Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice.Int J Mol Sci. 2021 Jul 2;22(13):7176. doi: 10.3390/ijms22137176. Int J Mol Sci. 2021. PMID: 34281229 Free PMC article.
-
A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren's Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses.Int J Mol Sci. 2022 May 29;23(11):6106. doi: 10.3390/ijms23116106. Int J Mol Sci. 2022. PMID: 35682784 Free PMC article.
-
New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model.Arch Oral Biol. 1999 May;44 Suppl 1:S21-5. doi: 10.1016/s0003-9969(99)00045-x. Arch Oral Biol. 1999. PMID: 10414851 Review.
-
MicroRNA in Sjögren's Syndrome: Their Potential Roles in Pathogenesis and Diagnosis.J Immunol Res. 2018 Jun 7;2018:7510174. doi: 10.1155/2018/7510174. eCollection 2018. J Immunol Res. 2018. PMID: 29977932 Free PMC article. Review.
Cited by
-
Marginal Zone B (MZB) Cells: Comparison of the Initial Identification of Immune Activity Leading to Dacryoadenitis and Sialadenitis in Experimental Sjögren's Syndrome.Int J Mol Sci. 2023 Jul 30;24(15):12209. doi: 10.3390/ijms241512209. Int J Mol Sci. 2023. PMID: 37569583 Free PMC article.
-
Integrated Bioinformatics and Validation Reveal Potential Biomarkers Associated With Progression of Primary Sjögren's Syndrome.Front Immunol. 2021 Jul 23;12:697157. doi: 10.3389/fimmu.2021.697157. eCollection 2021. Front Immunol. 2021. PMID: 34367157 Free PMC article.
-
CXCL13: a common target for immune-mediated inflammatory diseases.Clin Exp Med. 2024 Oct 24;24(1):244. doi: 10.1007/s10238-024-01508-8. Clin Exp Med. 2024. PMID: 39443356 Free PMC article. Review.
-
Upregulated Chemokine and Rho-GTPase Genes Define Immune Cell Emigration into Salivary Glands of Sjögren's Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice.Int J Mol Sci. 2021 Jul 2;22(13):7176. doi: 10.3390/ijms22137176. Int J Mol Sci. 2021. PMID: 34281229 Free PMC article.
-
Metabolic changes during evolution of Sjögren's in both an animal model and human patients.Heliyon. 2024 Dec 11;11(1):e41082. doi: 10.1016/j.heliyon.2024.e41082. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39801970 Free PMC article.
References
-
- Dörner T. Crossroads of B cell activation in autoimmunity: Rationale of targeting B cells. J. Rheumatol. Suppl. 2006;77:3–11. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

