Molecular Characterization of Velogenic Newcastle Disease Virus (Sub-Genotype VII.1.1) from Wild Birds, with Assessment of Its Pathogenicity in Susceptible Chickens
- PMID: 33672003
- PMCID: PMC7919289
- DOI: 10.3390/ani11020505
Molecular Characterization of Velogenic Newcastle Disease Virus (Sub-Genotype VII.1.1) from Wild Birds, with Assessment of Its Pathogenicity in Susceptible Chickens
Abstract
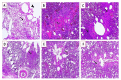
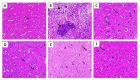
Newcastle disease (ND) is considered to be one of the most economically significant avian viral diseases. It has a worldwide distribution and a continuous diversity of genotypes. Despite its limited zoonotic potential, Newcastle disease virus (NDV) outbreaks in Egypt occur frequently and result in serious economic losses in the poultry industry. In this study, we investigated and characterized NDV in wild cattle egrets and house sparrows. Fifty cattle egrets and fifty house sparrows were collected from the vicinity of chicken farms in Kafrelsheikh Governorate, Egypt, which has a history of NDV infection. Lung, spleen, and brain tissue samples were pooled from each bird and screened for NDV by real-time reverse transcriptase polymerase chain reaction (RRT-PCR) and reverse transcriptase polymerase chain reaction (RT-PCR) to amplify the 370 bp NDV F gene fragment. NDV was detected by RRT-PCR in 22 of 50 (44%) cattle egrets and 13 of 50 (26%) house sparrows, while the conventional RT-PCR detected NDV in 18 of 50 (36%) cattle egrets and 10 of 50 (20%) of house sparrows. Phylogenic analysis revealed that the NDV strains identified in the present study are closely related to other Egyptian class II, sub-genotype VII.1.1 NDV strains from GenBank, having 99.7-98.5% identity. The pathogenicity of the wild-bird-origin NDV sub-genotype VII.1.1 NDV strains were assessed by experimental inoculation of identified strains (KFS-Motobas-2, KFS-Elhamoul-1, and KFS-Elhamoul-3) in 28-day-old specific-pathogen-free (SPF) Cobb chickens. The clinical signs and post-mortem changes of velogenic NDV genotype VII (GVII) were observed in inoculated chickens 3 to 7 days post-inoculation, with 67.5-70% mortality rates. NDV was detected in all NDV-inoculated chickens by RRT-PCR and RT-PCR at 3, 7, and 10 days post-inoculation. The histopathological findings of the experimentally infected chickens showed marked pulmonary congestion and pneumonia associated with complete bronchial stenosis. The spleen showed histocytic cell proliferation with marked lymphoid depletion, while the brain had malacia and diffuse gliosis. These findings provide interesting data about the characterization of NDV in wild birds from Egypt and add to our understanding of their possible role in the transmission dynamics of the disease in Egypt. Further research is needed to explore the role of other species of wild birds in the epidemiology of this disease and to compare the strains circulating in wild birds with those found in poultry.
Keywords: Egypt; NDV sub-genotype VII.1.1; RT-PCR; phylogenetic analysis; wild birds.
Conflict of interest statement
The authors declare no conflict of interest that can potentially influence the results of this study.
Figures







Similar articles
-
Pathogenesis of Velogenic Genotype VII.1.1 Newcastle Disease Virus Isolated from Chicken in Egypt via Different Inoculation Routes: Molecular, Histopathological, and Immunohistochemical Study.Animals (Basel). 2021 Dec 15;11(12):3567. doi: 10.3390/ani11123567. Animals (Basel). 2021. PMID: 34944344 Free PMC article.
-
Newcastle Disease Genotype VII Prevalence in Poultry and Wild Birds in Egypt.Viruses. 2022 Oct 13;14(10):2244. doi: 10.3390/v14102244. Viruses. 2022. PMID: 36298799 Free PMC article.
-
Biological characterization of wild-bird-origin avian avulavirus 1 and efficacy of currently applied vaccines against potential infection in commercial poultry.Arch Virol. 2018 Oct;163(10):2743-2755. doi: 10.1007/s00705-018-3916-5. Epub 2018 Jun 19. Arch Virol. 2018. PMID: 29922856
-
Newcastle disease: evolution of genotypes and the related diagnostic challenges.Infect Genet Evol. 2010 Jan;10(1):26-35. doi: 10.1016/j.meegid.2009.09.012. Epub 2009 Sep 30. Infect Genet Evol. 2010. PMID: 19800028 Review.
-
Cormorants as a Potentially Important Reservoir and Carrier of Newcastle Disease Virus on the Asian Continent.Front Vet Sci. 2021 Jun 14;8:648091. doi: 10.3389/fvets.2021.648091. eCollection 2021. Front Vet Sci. 2021. PMID: 34195243 Free PMC article. Review.
Cited by
-
Phylogenetic Analysis of Newcastle Disease Virus Isolated from Poultry in Live Bird Markets and Wild Waterfowl in Zambia.Microorganisms. 2024 Feb 8;12(2):354. doi: 10.3390/microorganisms12020354. Microorganisms. 2024. PMID: 38399757 Free PMC article.
-
Detection of Avian Orthoavulavirus-1 genotypes VI.2.1 and VII.1.1 with neuro-viscerotropic tropism in some backyard pigeons (Columbidae) in Eastern Saudi Arabia.Front Vet Sci. 2024 Feb 27;11:1352636. doi: 10.3389/fvets.2024.1352636. eCollection 2024. Front Vet Sci. 2024. PMID: 38500603 Free PMC article.
-
Genetic and antigenic characteristics of genotype VII.1.1 Newcastle disease viruses currently circulating in Ethiopian chickens.Virol J. 2025 Mar 6;22(1):63. doi: 10.1186/s12985-025-02686-x. Virol J. 2025. PMID: 40050904 Free PMC article.
-
Evaluation of the Newcastle disease virus genotype VII-mismatched vaccines in SPF chickens: A challenge efficacy study.Vet Anim Sci. 2024 Mar 28;24:100348. doi: 10.1016/j.vas.2024.100348. eCollection 2024 Jun. Vet Anim Sci. 2024. PMID: 38623086 Free PMC article.
-
Epidemiological study of Newcastle disease in chicken farms in China, 2019-2022.Front Vet Sci. 2024 Oct 30;11:1410878. doi: 10.3389/fvets.2024.1410878. eCollection 2024. Front Vet Sci. 2024. PMID: 39545258 Free PMC article.
References
-
- OIE World Animal Health Information Database (WAHIS Interface)—Version 1. [(accessed on 12 December 2020)]; Available online: http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home.
-
- . World Livestock Disease Atlas: A Quantitative Analysis of GlobalAnimal Health Data A (2006–2009) The World Bank for Reconstruction and Development, The World Bank ad TAFS Forum; Washington, DC, USA: 2011.
-
- Miller P.J., Koch G. Newcastle disease. Dis. Poult. 2013;13:89–138.
LinkOut - more resources
Full Text Sources
Other Literature Sources

