Versatile Reactivity of MnII Complexes in Reactions with N-Donor Heterocycles: Metamorphosis of Labile Homometallic Pivalates vs. Assembling of Endurable Heterometallic Acetates
- PMID: 33672016
- PMCID: PMC7919295
- DOI: 10.3390/molecules26041021
Versatile Reactivity of MnII Complexes in Reactions with N-Donor Heterocycles: Metamorphosis of Labile Homometallic Pivalates vs. Assembling of Endurable Heterometallic Acetates
Abstract
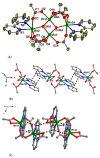
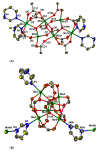
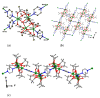
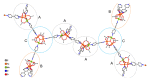
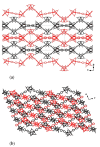
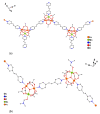
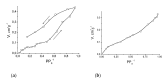
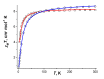
Reaction of 2,2'-bipyridine (2,2'-bipy) or 1,10-phenantroline (phen) with [Mn(Piv)2(EtOH)]n led to the formation of binuclear complexes [Mn2(Piv)4L2] (L = 2,2'-bipy (1), phen (2); Piv- is the anion of pivalic acid). Oxidation of 1 or 2 by air oxygen resulted in the formation of tetranuclear MnII/III complexes [Mn4O2(Piv)6L2] (L = 2,2'-bipy (3), phen (4)). The hexanuclear complex [Mn6(OH)2(Piv)10(pym)4] (5) was formed in the reaction of [Mn(Piv)2(EtOH)]n with pyrimidine (pym), while oxidation of 5 produced the coordination polymer [Mn6O2(Piv)10(pym)2]n (6). Use of pyrazine (pz) instead of pyrimidine led to the 2D-coordination polymer [Mn4(OH)(Piv)7(µ2-pz)2]n (7). Interaction of [Mn(Piv)2(EtOH)]n with FeCl3 resulted in the formation of the hexanuclear complex [MnII4FeIII2O2(Piv)10(MeCN)2(HPiv)2] (8). The reactions of [MnFe2O(OAc)6(H2O)3] with 4,4'-bipyridine (4,4'-bipy) or trans-1,2-(4-pyridyl)ethylene (bpe) led to the formation of 1D-polymers [MnFe2O(OAc)6L2]n·2nDMF, where L = 4,4'-bipy (9·2DMF), bpe (10·2DMF) and [MnFe2O(OAc)6(bpe)(DMF)]n·3.5nDMF (11·3.5DMF). All complexes were characterized by single-crystal X-ray diffraction. Desolvation of 11·3.5DMF led to a collapse of the porous crystal lattice that was confirmed by PXRD and N2 sorption measurements, while alcohol adsorption led to porous structure restoration. Weak antiferromagnetic exchange was found in the case of binuclear MnII complexes (JMn-Mn = -1.03 cm-1 for 1 and 2). According to magnetic data analysis (JMn-Mn = -(2.69 ÷ 0.42) cm-1) and DFT calculations (JMn-Mn = -(6.9 ÷ 0.9) cm-1) weak antiferromagnetic coupling between MnII ions also occurred in the tetranuclear {Mn4(OH)(Piv)7} unit of the 2D polymer 7. In contrast, strong antiferromagnetic coupling was found in oxo-bridged trinuclear fragment {MnFe2O(OAc)6} in 11·3.5DMF (JFe-Fe = -57.8 cm-1, JFe-Mn = -20.12 cm-1).
Keywords: coordination polymers; magnetic properties; manganese; polynuclear complexes; porous materials; pyridines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Glaser F., Wenger O.S. Recent Progress in the Development of Transition-Metal Based Photoredox Catalysts. Coord. Chem. Rev. 2020;405:213129. doi: 10.1016/j.ccr.2019.213129. - DOI
-
- Cooper B.G., Napoline J.W., Thomas C.M. Catalytic Applications of Early/Late Heterobimetallic Complexes. Cat. Rev. 2012;54:1–40. doi: 10.1080/01614940.2012.619931. - DOI
-
- Bilyachenko A.N., Dronova M.S., Yalymov A.I., Lamaty F., Bantreil X., Martinez J., Bizet C., Shul’pina L.S., Korlyukov A.A., Arkhipov D.E., et al. Cage-like Copper(II) Silsesquioxanes: Transmetalation Reactions and Structural, Quantum Chemical, and Catalytic Studies. Chem. Eur. J. 2015;21:8758–8770. doi: 10.1002/chem.201500791. - DOI - PubMed
-
- Kirillov A.M., Kirillova M.V., Pombeiro A.J.L. Homogeneous Multicopper Catalysts for Oxidation and Hydrocarboxylation of Alkanes. Adv. Inorg. Chem. 2013;65:1–31. doi: 10.1016/B978-0-12-404582-8.00001-8. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

