Chimeric Virus-Like Particles of Prawn Nodavirus Displaying Hepatitis B Virus Immunodominant Region: Biophysical Properties and Cytokine Response
- PMID: 33672018
- PMCID: PMC7919259
- DOI: 10.3390/ijms22041922
Chimeric Virus-Like Particles of Prawn Nodavirus Displaying Hepatitis B Virus Immunodominant Region: Biophysical Properties and Cytokine Response
Abstract
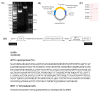
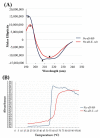
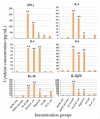
Hepatitis B is a major global health challenge. In the absence of an effective treatment for the disease, hepatitis B vaccines provide protection against the viral infection. However, some individuals do not have positive immune responses after being vaccinated with the hepatitis B vaccines available in the market. Thus, it is important to develop a more protective vaccine. Previously, we showed that hepatitis B virus (HBV) 'a' determinant (aD) displayed on the prawn nodavirus capsid (Nc) and expressed in Spodoptera frugiperda (Sf9) cells (namely, Nc-aD-Sf9) self-assembled into virus-like particles (VLPs). Immunisation of BALB/c mice with the Nc-aD-Sf9 VLPs showed significant induction of humoral, cellular and memory B-cell immunity. In the present study, the biophysical properties of the Nc-aD-Sf9 VLPs were studied using dynamic light scattering (DLS) and circular dichroism (CD) spectroscopy. Enzyme-linked immunosorbent assay (ELISA) was used to determine the antigenicity of the Nc-aD-Sf9 VLPs, and multiplex ELISA was employed to quantify the cytokine response induced by the VLPs administered intramuscularly into BALB/c mice (n = 8). CD spectroscopy of Nc-aD-Sf9 VLPs showed that the secondary structure of the VLPs predominantly consisted of beta (β)-sheets (44.8%), and they were thermally stable up to ~52 °C. ELISA revealed that the aD epitope of the VLPs was significantly antigenic to anti-HBV surface antigen (HBsAg) antibodies. In addition, multiplex ELISA of serum samples from the vaccinated mice showed a significant induction (p < 0.001) of IFN-γ, IL-4, IL-5, IL-6, IL-10, and IL-12p70. This cytokine profile is indicative of natural killer cell, macrophage, dendritic cell and cytotoxic T-lymphocyte activities, which suggests a prophylactic innate and adaptive cellular immune response mediated by Nc-aD-Sf9 VLPs. Interestingly, Nc-aD-Sf9 induced a more robust release of the aforementioned cytokines than that of Nc-aD VLPs produced in Escherichia coli and a commercially used hepatitis B vaccine. Overall, Nc-aD-Sf9 VLPs are thermally stable and significantly antigenic, demonstrating their potential as an HBV vaccine candidate.
Keywords: ELISA; Sf9 cells; capsid protein; circular dichroism; cytokine; hepatitis B virus; prawn nodavirus; virus-like particles; ‘a’ determinant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Induction of humoral and cell-mediated immune responses by hepatitis B virus epitope displayed on the virus-like particles of prawn nodavirus.Appl Environ Microbiol. 2015 Feb;81(3):882-9. doi: 10.1128/AEM.03695-14. Epub 2014 Nov 21. Appl Environ Microbiol. 2015. PMID: 25416760 Free PMC article.
-
Immunological Analysis of the Hepatitis B Virus "a" Determinant Displayed on Chimeric Virus-Like Particles of Macrobrachium rosenbergii Nodavirus Capsid Protein Produced in Sf9 Cells.Vaccines (Basel). 2020 Jun 4;8(2):275. doi: 10.3390/vaccines8020275. Vaccines (Basel). 2020. PMID: 32512923 Free PMC article.
-
Virus-like particle of Macrobrachium rosenbergii nodavirus produced in Spodoptera frugiperda (Sf9) cells is distinctive from that produced in Escherichia coli.Biotechnol Prog. 2017 Mar;33(2):549-557. doi: 10.1002/btpr.2409. Epub 2016 Nov 29. Biotechnol Prog. 2017. PMID: 27860432
-
Expression and characterization of hepatitis E virus-like particles and non-virus-like particles from insect cells.Biotechnol Appl Biochem. 2016 May;63(3):362-70. doi: 10.1002/bab.1379. Epub 2015 Jul 6. Biotechnol Appl Biochem. 2016. PMID: 25824972 Review.
-
DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response.Ann N Y Acad Sci. 1995 Nov 27;772:64-76. doi: 10.1111/j.1749-6632.1995.tb44732.x. Ann N Y Acad Sci. 1995. PMID: 8546414 Review.
Cited by
-
Virus-like Particles: Fundamentals and Biomedical Applications.Int J Mol Sci. 2022 Aug 2;23(15):8579. doi: 10.3390/ijms23158579. Int J Mol Sci. 2022. PMID: 35955711 Free PMC article. Review.
-
Advances in Nanoparticle Drug Delivery Systems for Anti-Hepatitis B Virus Therapy: A Narrative Review.Int J Mol Sci. 2021 Oct 18;22(20):11227. doi: 10.3390/ijms222011227. Int J Mol Sci. 2021. PMID: 34681886 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

