Supercritical Fluid Extract of Putranjiva roxburghii Wall. Seeds Mitigates Fertility Impairment in a Zebrafish Model
- PMID: 33672019
- PMCID: PMC7919291
- DOI: 10.3390/molecules26041020
Supercritical Fluid Extract of Putranjiva roxburghii Wall. Seeds Mitigates Fertility Impairment in a Zebrafish Model
Abstract
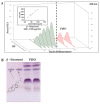
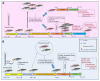
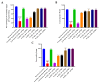
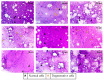
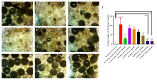
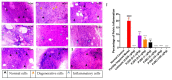
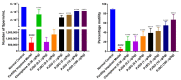
Putrajeevak (Putranjiva roxburghii Wall.; synonym Drypetes roxburghii (Wall.) Hurus) seeds have been used since ancient times in the treatment of infertility in the Ayurvedic system of medicine in India. In this study, the oil component of Putrajeevak seeds (PJSO) was extracted using the supercritical fluid extraction (SCFE) method using liquid CO2 and the constituents were analyzed using gas chromatography-flame ionized detectorand high-performance thin-layer chromatography. PJSO contained trace amounts of β-sitosterol with oleic and linoleic acids as the major fatty acid constituents. Male and female zebrafish were mutagenized with N-ethyl-N-nitrosourea (ENU) and fish that produced less than 20 viable embryos were selected for the study. SCFE oil extracts from the P. roxburghii seeds were used in this study to reverse fertility impairment. The mutant fish were fed with PJSO for a period of 14 days and the rates of fertility, conception, and fecundity were determined with wild-type healthy fish as a breeding partner. Treatment with PJSO increased the ovarian follicle count as well as the number of mature eggs, while reducing the number of ovarian cysts. Sperm count as well as sperm motility were greatly enhanced in the ENU-mutagenized male zebrafish when treated with PJSO. The results obtained in this study demonstrate the effectiveness of P. roxburghii seed oil in reversing impaired fertility in both male and female zebrafish models.
Keywords: N-ethyl-N-nitrosourea mutagenesis; Putrajeevak seed oil; Putranjiva roxburghii; follicle development; impaired fertility; sperm motility; zebrafish; β-sitosterol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Khare C.P. Indian Medicinal Plants. Springer; New York, NY, USA: 2007. Putranjiva roxburghii Wall; pp. 528–529.
-
- Minj E., Britto J.S. Pharmacognostic Studies of Putranjiva roxburghii Wall.(Putranjivaeceae) Int. J. Pharmacogn. Phytochem. Res. 2018;9 doi: 10.25258/phyto.v9i07.11177. - DOI
-
- Sánchez-Camargo A.P., Mendiola J.A., Ibáñez E., Herrero M. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Elsevier; Amsterdam, The Netherlands: 2014. Supercritical Fluid Extraction.
-
- Pereira C.G., Meireles M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2010;3:340–372. doi: 10.1007/s11947-009-0263-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

