Qualitative and Quantitative Comparison of Plasma Exosomes from Neonates and Adults
- PMID: 33672065
- PMCID: PMC7919666
- DOI: 10.3390/ijms22041926
Qualitative and Quantitative Comparison of Plasma Exosomes from Neonates and Adults
Abstract
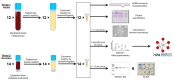
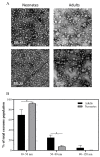
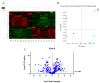
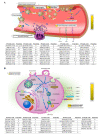
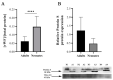
Exosomes are extracellular vesicles that contain nucleic acids, lipids and metabolites, and play a critical role in health and disease as mediators of intercellular communication. The majority of extracellular vesicles in the blood are platelet-derived. Compared to adults, neonatal platelets are hyporeactive and show impaired granule release, associated with defects in Soluble N-ethylmaleimide-sensitive fusion Attachment protein REceptor (SNARE) proteins. Since these proteins participate in biogenesis of exosomes, we investigated the potential differences between newborn and adult plasma-derived exosomes. Plasma-derived exosomes were isolated by ultracentrifugation of umbilical cord blood from full-term neonates or peripheral blood from adults. Exosome characterization included size determination by transmission electron microscopy and quantitative proteomic analysis. Plasma-derived exosomes from neonates were significantly smaller and contained 65% less protein than those from adults. Remarkably, 131 proteins were found to be differentially expressed, 83 overexpressed and 48 underexpressed in neonatal (vs. adult) exosomes. Whereas the upregulated proteins in plasma exosomes from neonates are associated with platelet activation, coagulation and granule secretion, most of the underexpressed proteins are immunoglobulins. This is the first study showing that exosome size and content change with age. Our findings may contribute to elucidating the potential "developmental hemostatic mismatch risk" associated with transfusions containing plasma exosomes from adults.
Keywords: exosomes; neonatal platelets; platelet transfusion; protein S; proteomic; von Willebrand factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

