Weakly Hydrated Anion Exchangers Doped with Cu2O and Cu0 Particles-Thermogravimetric Studies
- PMID: 33672076
- PMCID: PMC7919690
- DOI: 10.3390/ma14040925
Weakly Hydrated Anion Exchangers Doped with Cu2O and Cu0 Particles-Thermogravimetric Studies
Abstract
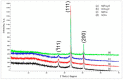
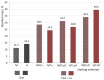
Hybrid ion exchangers (HIXs) containing fine Cu2O and Cu0 particles were subjected to thermal analysis in order to determine their hygroscopic water content (with regard to their anomalously low porosity) and to determine the effect of the oxidation state of the copper atom in the deposit on the thermal properties of composite materials. Commercially available anion exchangers, Amberlite IRA 900Cl (macroreticular, M) and Amberlite IRA 402OH (gel-like, G), were used as supporting materials. M/Cu2O, G/Cu2O, M/Cu and G/Cu, containing 4.3-8.4 wt% Cu, were subjected to thermal analysis under respectively air and N2. TG/DTG curves revealed that dry M/Cu and G/Cu contained as little as 7.2% and 4.3% hygroscopic water, while M/Cu2O and G/Cu2O contained respectively 10.6% and 9.4% (Cu0 was a stronger water repellent than Cu2O). The oxidation state of the copper atom in the deposit was found to affect the amount of the forming char, and also Cu0 was found to contribute to the formation of more char than in the pyrolysis of the pure resin (the anion exchanger with no copper deposit). Under air the two kinds of particles transformed into CuO, while under N2 metallic copper and char (from the resin phase) made up the solid residue. This means that in the pyrolysis of the HIXs the inorganic phase participated in char formation and it also transformed itself (undergoing reduction when possible). The above findings provide a basis for in-depth research aimed at the innovative use of copper-containing HIXs and at obtaining usable composite materials with a designed (organic-inorganic) composition.
Keywords: cuprous oxide; hybrid ion exchanger; pyrolysis; thermal analysis; zero valent copper.
Conflict of interest statement
Authors declare no conflict of interest.
Figures






References
-
- Wang J., Wan Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog. Nucl. Energy. 2015;78:47–55. doi: 10.1016/j.pnucene.2014.08.003. - DOI
-
- Choi W.N., Lee U., Kim H.R. Radiological assessment on spent resin treatment facility and transportation for radioactive waste disposal. Prog. Nucl. Energy. 2020;118:103125. doi: 10.1016/j.pnucene.2019.103125. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

