Role of Endoscopic Ultrasound in the Diagnosis of Pancreatic Neuroendocrine Neoplasms
- PMID: 33672085
- PMCID: PMC7919683
- DOI: 10.3390/diagnostics11020316
Role of Endoscopic Ultrasound in the Diagnosis of Pancreatic Neuroendocrine Neoplasms
Abstract
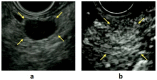
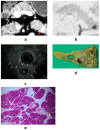
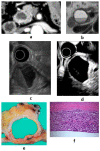
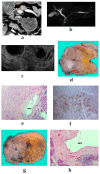
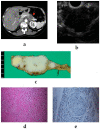
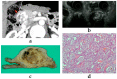
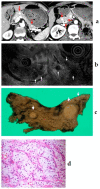
Although pancreatic neuroendocrine neoplasms (PNENs) are relatively rare tumors, their number is increasing with advances in diagnostic imaging modalities. Even small lesions that are difficult to detect using computed tomography or magnetic resonance imaging can now be detected with endoscopic ultrasound (EUS). Contrast-enhanced EUS is useful, and not only diagnosis but also malignancy detection has become possible by evaluating the vascularity of tumors. Pathological diagnosis using EUS with fine-needle aspiration (EUS-FNA) is useful when diagnostic imaging is difficult. EUS-FNA can also be used to evaluate the grade of malignancy. Pooling the data of the studies that compared the PNENs grading between EUS-FNA samples and surgical specimens showed a concordance rate of 77.5% (κ-statistic = 0.65, 95% confidence interval = 0.59-0.71, p < 0.01). Stratified analysis for small tumor size (2 cm) showed that the concordance rate was 84.5% and the kappa correlation index was 0.59 (95% confidence interval = 0.43-0.74, p < 0.01). The evolution of ultrasound imaging technologies such as contrast-enhanced and elastography and the artificial intelligence that analyzes them, the evolution of needles, and genetic analysis, will further develop the diagnosis and treatment of PNENs in the future.
Keywords: endoscopic ultrasound; pancreatic neuroendocrine neoplasms; pancreatic tumor.
Conflict of interest statement
A.K. received honoraria as a lecture fee from Olympus Co., Tokyo, Japan. The sponsors had no role in the design, execution, interpretation, or writing of the study. The other authors declare no conflicts of interest relevant to this article.
Figures
References
-
- Katanuma A., Maguchi H., Osanai M., Takahashi K. The difference in the capability of delineation between convex and radial arrayed echoendoscope for pancreas and biliary tract; case reports from the standpoint of both convex and radial arrayed echoendoscope. Dig. Endosc. 2011;23:2–8. doi: 10.1111/j.1443-1661.2011.01131.x. - DOI - PubMed
-
- Kaneko M., Katanuma A., Maguchi H., Takahashi K., Osanai M., Yane K., Hashigo S., Harada R., Kato S., Kato R. Prospective, randomized, comparative study of delineation capability of radial scanning and curved linear array endoscopic ultrasound for the pancreaticobiliary region. Endosc. Int. Open. 2014;2:E160. doi: 10.1055/s-0034-1377384. - DOI - PMC - PubMed
-
- Puli S.R., Kalva N., Bechtold M.L., Pamulaparthy S.R., Cashman M.D., Estes N.C., Pearl R.H., Volmar F.-H., Dillon S., Shekleton M.F. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: A systematic review and meta analysis. World J. Gastroenterol. WJG. 2013;19:3678. doi: 10.3748/wjg.v19.i23.3678. - DOI - PMC - PubMed
-
- Manta R., Nardi E., Pagano N., Ricci C., Sica M., Castellani D., Bertani H., Piccoli M., Mullineris B., Tringali A. Pre-operative diagnosis of pancreatic neuroendocrine tumors with endoscopic ultrasonography and computed tomography in a large series. J. Gastrointestin. Liver Dis. 2016;25:317–321. doi: 10.15403/jgld.2014.1121.253.ned. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

