Assessment of Exposure to Mycotoxins in Spanish Children through the Analysis of Their Levels in Plasma Samples
- PMID: 33672088
- PMCID: PMC7919644
- DOI: 10.3390/toxins13020150
Assessment of Exposure to Mycotoxins in Spanish Children through the Analysis of Their Levels in Plasma Samples
Abstract
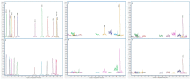
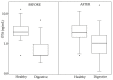
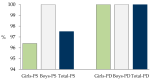
In this study, we present, for the first time in Spain, the levels of 19 mycotoxins in plasma samples from healthy and sick children (digestive, autism spectrum (ASD), and attention deficit hyperactivity (ADHD) disorders) (n = 79, aged 2-16). The samples were analyzed by liquid chromatography-mass spectrometry (triple quadrupole) (LC-MS/MS). To detect Phase II metabolites, the samples were reanalyzed after pre-treatment with β-glucuronidase/arylsulfatase. The most prevalent mycotoxin was ochratoxin A (OTA) in all groups of children, before and after enzyme treatment. In healthy children, the incidence of OTA was 92.5% in both cases and higher than in sick children before (36.7% in digestive disorders, 50% in ASD, and 14.3% in ADHD) and also after the enzymatic treatment (76.6 % in digestive disorders, 50% in ASD, and 85.7% in ADHD). OTA levels increased in over 40% of healthy children after enzymatic treatment, and this increase in incidence and levels was also observed in all sick children. This suggests the presence of OTA conjugates in plasma. In addition, differences in OTA metabolism may be assumed. OTA levels are higher in healthy children, even after enzymatic treatment (mean OTA value for healthy children 3.29 ng/mL, 1.90 ng/mL for digestive disorders, 1.90 ng/mL for ASD, and 0.82 ng/mL for ADHD). Ochratoxin B appears only in the samples of healthy children with a low incidence (11.4%), always co-occurring with OTA. Sterigmatocystin (STER) was detected after enzymatic hydrolysis with a high incidence in all groups, especially in sick children (98.7% in healthy children and 100% in patients). This supports glucuronidation as a pathway for STER metabolism in children. Although other mycotoxins were studied (aflatoxins B1, B2, G1, G2, and M1; T-2 and HT-2 toxins; deoxynivalenol, deepoxy-deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol; zearalenone; nivalenol; fusarenon-X; neosolaniol; and diacetoxyscirpenol), they were not detected either before or after enzymatic treatment in any of the groups of children. In conclusion, OTA and STER should be highly considered in the risk assessment of mycotoxins. Studies concerning their sources of exposure, toxicokinetics, and the relationship between plasma levels and toxic effects are of utmost importance in children.
Keywords: child; digestive problems; human biomonitoring; mycotoxins; ochratoxin A; ochratoxin B; sterigmatocystin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Biomonitoring of Mycotoxins in Plasma of Patients with Alzheimer's and Parkinson's Disease.Toxins (Basel). 2021 Jul 10;13(7):477. doi: 10.3390/toxins13070477. Toxins (Basel). 2021. PMID: 34357949 Free PMC article.
-
Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain.Toxins (Basel). 2020 Nov 27;12(12):750. doi: 10.3390/toxins12120750. Toxins (Basel). 2020. PMID: 33261074 Free PMC article.
-
Development and validation of a methodology based on Captiva EMR-lipid clean-up and LC-MS/MS analysis for the simultaneous determination of mycotoxins in human plasma.Talanta. 2020 Jan 1;206:120193. doi: 10.1016/j.talanta.2019.120193. Epub 2019 Jul 30. Talanta. 2020. PMID: 31514835
-
Studies on the Presence of Mycotoxins in Biological Samples: An Overview.Toxins (Basel). 2017 Aug 18;9(8):251. doi: 10.3390/toxins9080251. Toxins (Basel). 2017. PMID: 28820481 Free PMC article. Review.
-
Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review.Toxins (Basel). 2020 Feb 27;12(3):147. doi: 10.3390/toxins12030147. Toxins (Basel). 2020. PMID: 32121036 Free PMC article.
Cited by
-
Mycotoxin Exposure during the First 1000 Days of Life and Its Impact on Children's Health: A Clinical Overview.Toxins (Basel). 2022 Mar 4;14(3):189. doi: 10.3390/toxins14030189. Toxins (Basel). 2022. PMID: 35324686 Free PMC article. Review.
-
Prevalence and health risk evaluations of mycotoxins in drinking water sources in Nigeria.RSC Adv. 2024 Oct 29;14(46):34435-34447. doi: 10.1039/d4ra04866k. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39473792 Free PMC article.
-
Application of cold atmospheric plasma for decontamination of toxigenic fungi and mycotoxins: a systematic review.Front Microbiol. 2025 Jan 3;15:1502915. doi: 10.3389/fmicb.2024.1502915. eCollection 2024. Front Microbiol. 2025. PMID: 39831113 Free PMC article.
-
High-Coverage UHPLC-MS/MS Analysis of 67 Mycotoxins in Plasma for Male Infertility Exposure Studies.Toxics. 2024 May 28;12(6):395. doi: 10.3390/toxics12060395. Toxics. 2024. PMID: 38922075 Free PMC article.
-
Mycotoxins of Concern in Children and Infant Cereal Food at European Level: Incidence and Bioaccessibility.Toxins (Basel). 2022 Jul 15;14(7):488. doi: 10.3390/toxins14070488. Toxins (Basel). 2022. PMID: 35878226 Free PMC article. Review.
References
-
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Kujawa M. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. WHO; Geneva, Switzerland: 1994. Volume 56—Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; p. 351.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

