Integrin Regulation in Immunological and Cancerous Cells and Exosomes
- PMID: 33672100
- PMCID: PMC7926977
- DOI: 10.3390/ijms22042193
Integrin Regulation in Immunological and Cancerous Cells and Exosomes
Abstract
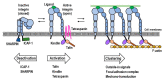
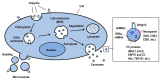
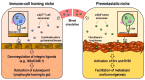
Integrins represent the biologically and medically significant family of cell adhesion molecules that govern a wide range of normal physiology. The activities of integrins in cells are dynamically controlled via activation-dependent conformational changes regulated by the balance of intracellular activators, such as talin and kindlin, and inactivators, such as Shank-associated RH domain interactor (SHARPIN) and integrin cytoplasmic domain-associated protein 1 (ICAP-1). The activities of integrins are alternatively controlled by homotypic lateral association with themselves to induce integrin clustering and/or by heterotypic lateral engagement with tetraspanin and syndecan in the same cells to modulate integrin adhesiveness. It has recently emerged that integrins are expressed not only in cells but also in exosomes, important entities of extracellular vesicles secreted from cells. Exosomal integrins have received considerable attention in recent years, and they are clearly involved in determining the tissue distribution of exosomes, forming premetastatic niches, supporting internalization of exosomes by target cells and mediating exosome-mediated transfer of the membrane proteins and associated kinases to target cells. A growing body of evidence shows that tumor and immune cell exosomes have the ability to alter endothelial characteristics (proliferation, migration) and gene expression, some of these effects being facilitated by vesicle-bound integrins. As endothelial metabolism is now thought to play a key role in tumor angiogenesis, we also discuss how tumor cells and their exosomes pleiotropically modulate endothelial functions in the tumor microenvironment.
Keywords: angiogenesis; endothelial metabolism; exosome; integrin; kindlin; talin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Talin-2 regulates integrin functions in exosomes.Biochem Biophys Res Commun. 2019 May 7;512(3):429-434. doi: 10.1016/j.bbrc.2019.03.027. Epub 2019 Mar 14. Biochem Biophys Res Commun. 2019. PMID: 30879762
-
Talin and kindlin cooperate to control the density of integrin clusters.J Cell Sci. 2023 Apr 15;136(8):jcs260746. doi: 10.1242/jcs.260746. Epub 2023 Apr 21. J Cell Sci. 2023. PMID: 37083041
-
Targeted remodeling of breast cancer and immune cell homing niches by exosomal integrins.Diagn Pathol. 2020 Apr 18;15(1):38. doi: 10.1186/s13000-020-00959-3. Diagn Pathol. 2020. PMID: 32305065 Free PMC article.
-
Integrin activation by talin, kindlin and mechanical forces.Nat Cell Biol. 2019 Jan;21(1):25-31. doi: 10.1038/s41556-018-0234-9. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602766 Review.
-
Kindlin: helper, co-activator, or booster of talin in integrin activation?Curr Opin Hematol. 2011 Sep;18(5):356-60. doi: 10.1097/MOH.0b013e3283497f09. Curr Opin Hematol. 2011. PMID: 21730832 Review.
Cited by
-
Tumour Derived Extracellular Vesicles: Challenging Target to Blunt Tumour Immune Evasion.Cancers (Basel). 2022 Aug 20;14(16):4020. doi: 10.3390/cancers14164020. Cancers (Basel). 2022. PMID: 36011012 Free PMC article. Review.
-
Exosome-powered neuropharmaceutics: unlocking the blood-brain barrier for next-gen therapies.J Nanobiotechnology. 2025 May 3;23(1):329. doi: 10.1186/s12951-025-03352-8. J Nanobiotechnology. 2025. PMID: 40319325 Free PMC article. Review.
-
Integrin β5 interacts with G3BP1 through activating FAK/Src signaling pathway to promote gastric carcinogenesis.Sci Rep. 2025 Aug 5;15(1):28633. doi: 10.1038/s41598-025-14067-z. Sci Rep. 2025. PMID: 40764649 Free PMC article.
-
Modelling the complex nature of the tumor microenvironment: 3D tumor spheroids as an evolving tool.J Biomed Sci. 2024 Jan 23;31(1):13. doi: 10.1186/s12929-024-00997-9. J Biomed Sci. 2024. PMID: 38254117 Free PMC article. Review.
-
Molecular Determinants Involved in the Docking and Uptake of Tumor-Derived Extracellular Vesicles: Implications in Cancer.Int J Mol Sci. 2024 Mar 19;25(6):3449. doi: 10.3390/ijms25063449. Int J Mol Sci. 2024. PMID: 38542421 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

