The Interplay between Drivers of Erythropoiesis and Iron Homeostasis in Rare Hereditary Anemias: Tipping the Balance
- PMID: 33672223
- PMCID: PMC7927117
- DOI: 10.3390/ijms22042204
The Interplay between Drivers of Erythropoiesis and Iron Homeostasis in Rare Hereditary Anemias: Tipping the Balance
Abstract
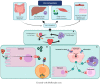
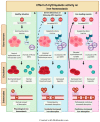
Rare hereditary anemias (RHA) represent a group of disorders characterized by either impaired production of erythrocytes or decreased survival (i.e., hemolysis). In RHA, the regulation of iron metabolism and erythropoiesis is often disturbed, leading to iron overload or worsening of chronic anemia due to unavailability of iron for erythropoiesis. Whereas iron overload generally is a well-recognized complication in patients requiring regular blood transfusions, it is also a significant problem in a large proportion of patients with RHA that are not transfusion dependent. This indicates that RHA share disease-specific defects in erythroid development that are linked to intrinsic defects in iron metabolism. In this review, we discuss the key regulators involved in the interplay between iron and erythropoiesis and their importance in the spectrum of RHA.
Keywords: erythropoiesis; ineffective erythropoiesis; iron metabolism; iron overload.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Piperno A. Classification and diagnosis of iron overload. Haematology. 1998;83:447–455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

