Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs
- PMID: 33672235
- PMCID: PMC7926383
- DOI: 10.3390/biology10020167
Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs
Abstract
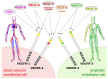
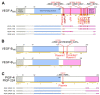
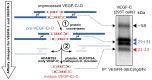
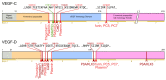
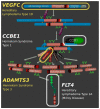
Specific proteolytic cleavages turn on, modify, or turn off the activity of vascular endothelial growth factors (VEGFs). Proteolysis is most prominent among the lymph-angiogenic VEGF-C and VEGF-D, which are synthesized as precursors that need to undergo enzymatic removal of their C- and N-terminal propeptides before they can activate their receptors. At least five different proteases mediate the activating cleavage of VEGF-C: plasmin, ADAMTS3, prostate-specific antigen, cathepsin D, and thrombin. All of these proteases except for ADAMTS3 can also activate VEGF-D. Processing by different proteases results in distinct forms of the "mature" growth factors, which differ in affinity and receptor activation potential. The "default" VEGF-C-activating enzyme ADAMTS3 does not activate VEGF-D, and therefore, VEGF-C and VEGF-D do function in different contexts. VEGF-C itself is also regulated in different contexts by distinct proteases. During embryonic development, ADAMTS3 activates VEGF-C. The other activating proteases are likely important for non-developmental lymphangiogenesis during, e.g., tissue regeneration, inflammation, immune response, and pathological tumor-associated lymphangiogenesis. The better we understand these events at the molecular level, the greater our chances of developing successful therapies targeting VEGF-C and VEGF-D for diseases involving the lymphatics such as lymphedema or cancer.
Keywords: ADAMTS3; CCBE1; KLK3; PlGF; VEGF-A; VEGF-B; VEGF-C; VEGF-D; angiogenesis; cathepsin D; lymphangiogenesis; lymphedema; metastasis; plasmin; prostate-specific antigen (PSA); proteases; proteolytic activation; thrombin; vascular biology; vascular endothelial growth factors (VEGFs); wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Meyer M., Clauss M., Lepple-Wienhues A., Waltenberger J., Augustin H.G., Ziche M., Lanz C., Büttner M., Rziha H.-J., Dehio C. A Novel Vascular Endothelial Growth Factor Encoded by Orf Virus, VEGF-E, Mediates Angiogenesis via Signalling through VEGFR-2 (KDR) but Not VEGFR-1 (Flt-1) Receptor Tyrosine Kinases. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. - DOI - PMC - PubMed
-
- Ogawa S., Oku A., Sawano A., Yamaguchi S., Yazaki Y., Shibuya M. A Novel Type of Vascular Endothelial Growth Factor, VEGF-E (NZ-7 VEGF), Preferentially Utilizes KDR/Flk-1 Receptor and Carries a Potent Mitotic Activity without Heparin-Binding Domain. J. Biol. Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

