Differential Effects of STCH and Stress-Inducible Hsp70 on the Stability and Maturation of NKCC2
- PMID: 33672238
- PMCID: PMC7926544
- DOI: 10.3390/ijms22042207
Differential Effects of STCH and Stress-Inducible Hsp70 on the Stability and Maturation of NKCC2
Abstract
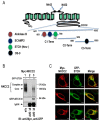
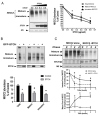
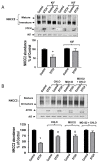
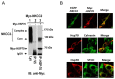
Mutations in the Na-K-2Cl co-transporter NKCC2 lead to type I Bartter syndrome, a life-threatening kidney disease. We previously showed that export from the ER constitutes the limiting step in NKCC2 maturation and cell surface expression. Yet, the molecular mechanisms involved in this process remain obscure. Here, we report the identification of chaperone stress 70 protein (STCH) and the stress-inducible heat shock protein 70 (Hsp70), as two novel binding partners of the ER-resident form of NKCC2. STCH knock-down increased total NKCC2 expression whereas Hsp70 knock-down or its inhibition by YM-01 had the opposite effect. Accordingly, overexpressing of STCH and Hsp70 exerted opposite actions on total protein abundance of NKCC2 and its folding mutants. Cycloheximide chase assay showed that in cells over-expressing STCH, NKCC2 stability and maturation are heavily impaired. In contrast to STCH, Hsp70 co-expression increased NKCC2 maturation. Interestingly, treatment by protein degradation inhibitors revealed that in addition to the proteasome, the ER associated degradation (ERAD) of NKCC2 mediated by STCH, involves also the ER-to-lysosome-associated degradation pathway. In summary, our data are consistent with STCH and Hsp70 having differential and antagonistic effects with regard to NKCC2 biogenesis. These findings may have an impact on our understanding and potential treatment of diseases related to aberrant NKCC2 trafficking and expression.
Keywords: Bartter syndrome; ERAD; Hsp70; NKCC2; STCH; hypertension; membrane; trafficking.
Conflict of interest statement
The authors declare that they have no conflict of interest with the content of this article.
Figures




References
-
- Gamba G., Miyanoshita A., Lombardi M., Lytton J., Lee W.S., Hediger M.A., Hebert S.C. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 1994;269:17713–17722. doi: 10.1016/S0021-9258(17)32499-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

