Oxidatively Modified LDL Suppresses Lymphangiogenesis via CD36 Signaling
- PMID: 33672291
- PMCID: PMC7926875
- DOI: 10.3390/antiox10020331
Oxidatively Modified LDL Suppresses Lymphangiogenesis via CD36 Signaling
Abstract
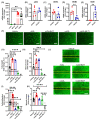
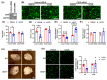
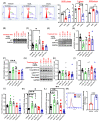
Arterial accumulation of plasma-derived LDL and its subsequent oxidation contributes to atherosclerosis. Lymphatic vessel (LV)-mediated removal of arterial cholesterol has been shown to reduce atherosclerotic lesion formation. However, the precise mechanisms that regulate LV density and function in atherosclerotic vessels remain to be identified. The aim of this study was to investigate the role of native LDL (nLDL) and oxidized LDL (oxLDL) in modulating lymphangiogenesis and underlying molecular mechanisms. Western blotting and immunostaining experiments demonstrated increased oxLDL expression in human atherosclerotic arteries. Furthermore, elevated oxLDL levels were detected in the adventitial layer, where LV are primarily present. Treatment of human lymphatic endothelial cells (LEC) with oxLDL inhibited in vitro tube formation, while nLDL stimulated it. Similar results were observed with Matrigel plug assay in vivo. CD36 deletion in mice and its siRNA-mediated knockdown in LEC prevented oxLDL-induced inhibition of lymphangiogenesis. In addition, oxLDL via CD36 receptor suppressed cell cycle, downregulated AKT and eNOS expression, and increased levels of p27 in LEC. Collectively, these results indicate that oxLDL inhibits lymphangiogenesis via CD36-mediated regulation of AKT/eNOS pathway and cell cycle. These findings suggest that therapeutic blockade of LEC CD36 may promote arterial lymphangiogenesis, leading to increased cholesterol removal from the arterial wall and reduced atherosclerosis.
Keywords: CD36; atherosclerosis; lymphangiogenesis; native LDL; oxidized LDL.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. - DOI - PMC - PubMed
-
- Rademakers T., Van Der Vorst E.P.C., Daissormont I.T.M.N., Otten J.J.T., Theodorou K., Theelen T.L., Gijbels M., Anisimov A., Nurmi H., Lindeman J.H.N., et al. Adventitial lymphatic capillary expansion impacts on plaque T cell accumulation in atherosclerosis. Sci. Rep. 2017;7:srep45263. doi: 10.1038/srep45263. - DOI - PMC - PubMed
-
- Vuorio T., Nurmi H., Moulton K., Kurkipuro J., Robciuc M.R., Öhman M., Heinonen S.E., Samaranayake H., Heikura T., Alitalo K., et al. Lymphatic Vessel Insufficiency in Hypercholesterolemic Mice Alters Lipoprotein Levels and Promotes Atherogenesis. Arter. Thromb. Vasc. Biol. 2014;34:1162–1170. doi: 10.1161/ATVBAHA.114.302528. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

