Actin Cytoskeleton and Regulation of TGFβ Signaling: Exploring Their Links
- PMID: 33672325
- PMCID: PMC7926735
- DOI: 10.3390/biom11020336
Actin Cytoskeleton and Regulation of TGFβ Signaling: Exploring Their Links
Abstract
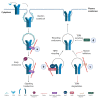
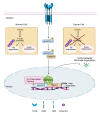
Human tissues, to maintain their architecture and function, respond to injuries by activating intricate biochemical and physical mechanisms that regulates intercellular communication crucial in maintaining tissue homeostasis. Coordination of the communication occurs through the activity of different actin cytoskeletal regulators, physically connected to extracellular matrix through integrins, generating a platform of biochemical and biomechanical signaling that is deregulated in cancer. Among the major pathways, a controller of cellular functions is the cytokine transforming growth factor β (TGFβ), which remains a complex and central signaling network still to be interpreted and explained in cancer progression. Here, we discuss the link between actin dynamics and TGFβ signaling with the aim of exploring their aberrant interaction in cancer.
Keywords: TGFβ; actin cytoskeleton; actin-binding proteins; extracellular matrix; tumor microenvironment.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

