Integrase-Defective Lentiviral Vector Is an Efficient Vaccine Platform for Cancer Immunotherapy
- PMID: 33672349
- PMCID: PMC7927015
- DOI: 10.3390/v13020355
Integrase-Defective Lentiviral Vector Is an Efficient Vaccine Platform for Cancer Immunotherapy
Abstract
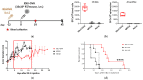
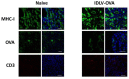
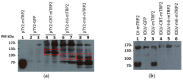
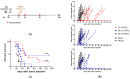
Integrase-defective lentiviral vectors (IDLVs) have been used as a safe and efficient delivery system in several immunization protocols in murine and non-human primate preclinical models as well as in recent clinical trials. In this work, we validated in preclinical murine models our vaccine platform based on IDLVs as delivery system for cancer immunotherapy. To evaluate the anti-tumor activity of our vaccine strategy we generated IDLV delivering ovalbumin (OVA) as a non-self-model antigen and TRP2 as a self-tumor associated antigen (TAA) of melanoma. Results demonstrated the ability of IDLVs to eradicate and/or controlling tumor growth after a single immunization in preventive and therapeutic approaches, using lymphoma and melanoma expressing OVA. Importantly, LV-TRP2 but not IDLV-TRP2 was able to break tolerance efficiently and prevent tumor growth of B16F10 melanoma cells. In order to improve the IDLV efficacy, the human homologue of murine TRP2 was used, showing the ability to break tolerance and control the tumor growth. These results validate the use of IDLV for cancer therapy.
Keywords: immune tolerance; immunotherapy; lentiviral vector: vaccine; tumor; tumor associated antigen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., Redfern C.H., Ferrari A.C., Dreicer R., Sims R.B., et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

