The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells
- PMID: 33672352
- PMCID: PMC7926403
- DOI: 10.3390/nano11020557
The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells
Abstract
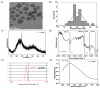
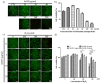
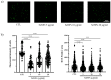
Reactive oxygen species (ROS) regulate various functions of cells, including cell death, viability, and differentiation, and nanoparticles influence ROS depending on their size and shape. Selenium is known to regulate various physiological functions, such as cell differentiations and anti-inflammatory functions, and plays an important role in the regulation of ROS as an antioxidant. This study aims to investigate the effect of selenium nanoparticles (SeNPs) on the differentiation of osteogenic MC3T3-E1 cells. After fabrication of SeNPs with a size of 25.3 ± 2.6 nm, and confirmation of its oxidase-like activity, SeNPs were added to MC3T3-E1 cells with or without H2O2: 5~20 μg/mL SeNPs recovered cells damaged by 200 μM H2O2 via the intracellular ROS downregulating role of SeNPs, revealed by the ROS staining assay. The increase in osteogenic maturation with SeNPs was gradually investigated by expression of osteogenic genes at 3 and 7 days, Alkaline phosphatase activity staining at 14 days, and Alizarin red S staining at 28 days. Therefore, the role of SeNPs in regulating ROS and their therapeutic effects on the differentiation of MC3T3-E1 cells were determined, leading to possible applications for bone treatment.
Keywords: MC3T3-E1; nanoparticles; osteogenic differentiation; reactive oxygen species; selenium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

