Modulation of Inflammatory Mediators by Polymeric Nanoparticles Loaded with Anti-Inflammatory Drugs
- PMID: 33672354
- PMCID: PMC7926915
- DOI: 10.3390/pharmaceutics13020290
Modulation of Inflammatory Mediators by Polymeric Nanoparticles Loaded with Anti-Inflammatory Drugs
Abstract
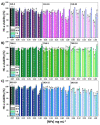
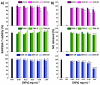
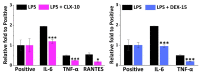
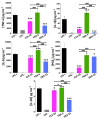
The first-line treatment of osteoarthritis is based on anti-inflammatory drugs, the most currently used being nonsteroidal anti-inflammatory drugs, selective cyclooxygenase 2 (COX-2) inhibitors and corticoids. Most of them present cytotoxicity and low bioavailability in physiological conditions, making necessary the administration of high drug concentrations causing several side effects. The goal of this work was to encapsulate three hydrophobic anti-inflammatory drugs of different natures (celecoxib, tenoxicam and dexamethasone) into core-shell terpolymer nanoparticles with potential applications in osteoarthritis. Nanoparticles presented hydrodynamic diameters between 110 and 130 nm and almost neutral surface charges (between -1 and -5 mV). Encapsulation efficiencies were highly dependent on the loaded drug and its water solubility, having higher values for celecoxib (39-72%) followed by tenoxicam (20-24%) and dexamethasone (14-26%). Nanoencapsulation reduced celecoxib and dexamethasone cytotoxicity in human articular chondrocytes and murine RAW264.7 macrophages. Moreover, the three loaded systems did not show cytotoxic effects in a wide range of concentrations. Celecoxib and dexamethasone-loaded nanoparticles reduced the release of different inflammatory mediators (NO, TNF-α, IL-1β, IL-6, PGE2 and IL-10) by lipopolysaccharide (LPS)-stimulated RAW264.7. Tenoxicam-loaded nanoparticles reduced NO and PGE2 production, although an overexpression of IL-1β, IL-6 and IL-10 was observed. Finally, all nanoparticles proved to be biocompatible in a subcutaneous injection model in rats. These findings suggest that these loaded nanoparticles could be suitable candidates for the treatment of inflammatory processes associated with osteoarthritis due to their demonstrated in vitro activity as regulators of inflammatory mediator production.
Keywords: celecoxib; dexamethasone; inflammatory mediators; nanoparticles; osteoarthritis; tenoxicam.
Conflict of interest statement
Gloria María Pontes-Quero is a doctoral PhD student financially supported by the company ALODIA Farmacéutica and Comunidad de Madrid. Juan Pérez is the CEO and founder of ALODIA Farmacéutica. ALODIA Farmacéutica had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Other authors declare no conflict of interest.
Figures


Similar articles
-
Dexamethasone microspheres and celecoxib microcrystals loaded into injectable gels for enhanced knee osteoarthritis therapy.Int J Pharm. 2022 Jun 25;622:121802. doi: 10.1016/j.ijpharm.2022.121802. Epub 2022 May 5. Int J Pharm. 2022. PMID: 35526699
-
Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression.Am J Transl Res. 2019 Nov 15;11(11):6775-6789. eCollection 2019. Am J Transl Res. 2019. PMID: 31814887 Free PMC article.
-
A combination of chitosan nanoparticles loaded with celecoxib and kartogenin has anti-inflammatory and chondroprotective effects: Results from an in vitro model of osteoarthritis.Heliyon. 2024 May 10;10(10):e31058. doi: 10.1016/j.heliyon.2024.e31058. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803939 Free PMC article.
-
Evaluation of the effect of polyphenol of escin compared with ibuprofen and dexamethasone in synoviocyte model for osteoarthritis: an in vitro study.Clin Rheumatol. 2018 Sep;37(9):2471-2478. doi: 10.1007/s10067-018-4097-z. Epub 2018 Apr 16. Clin Rheumatol. 2018. PMID: 29663159
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
Cited by
-
Polymeric Nanoparticles for Drug Delivery in Osteoarthritis.Pharmaceutics. 2022 Nov 29;14(12):2639. doi: 10.3390/pharmaceutics14122639. Pharmaceutics. 2022. PMID: 36559133 Free PMC article. Review.
-
Single-cell analysis reveals that Jinwu Gutong capsule attenuates the inflammatory activity of synovial cells in osteoarthritis by inhibiting AKR1C3.Front Physiol. 2022 Nov 23;13:1031996. doi: 10.3389/fphys.2022.1031996. eCollection 2022. Front Physiol. 2022. PMID: 36505054 Free PMC article.
-
The Role of Polymeric Biomaterials in the Treatment of Articular Osteoarthritis.Pharmaceutics. 2022 Aug 6;14(8):1644. doi: 10.3390/pharmaceutics14081644. Pharmaceutics. 2022. PMID: 36015270 Free PMC article. Review.
-
Emodin ameliorates acute pancreatitis-induced lung injury by suppressing NLRP3 inflammasome-mediated neutrophil recruitment.Exp Ther Med. 2021 Aug;22(2):857. doi: 10.3892/etm.2021.10289. Epub 2021 Jun 9. Exp Ther Med. 2021. PMID: 34178130 Free PMC article.
-
Chemopreventive and Biological Strategies in the Management of Oral Potentially Malignant and Malignant Disorders.Bioengineering (Basel). 2024 Jan 9;11(1):65. doi: 10.3390/bioengineering11010065. Bioengineering (Basel). 2024. PMID: 38247942 Free PMC article. Review.
References
-
- Minguzzi M., Cetrullo S., D’Adamo S., Silvestri Y., Flamigni F., Borzi R.M. Emerging Players at the Intersection of Chondrocyte Loss of Maturational Arrest, Oxidative Stress, Senescence and Low-Grade Inflammation in Osteoarthritis. Oxid. Med. Cell. Longev. 2018;2018:3075293. doi: 10.1155/2018/3075293. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

