The 1β-Hydroxy-Deoxycholic Acid to Deoxycholic Acid Urinary Metabolic Ratio: Toward a Phenotyping of CYP3A Using an Endogenous Marker?
- PMID: 33672438
- PMCID: PMC7923269
- DOI: 10.3390/jpm11020150
The 1β-Hydroxy-Deoxycholic Acid to Deoxycholic Acid Urinary Metabolic Ratio: Toward a Phenotyping of CYP3A Using an Endogenous Marker?
Abstract
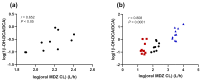
In this study, we assessed the potential use of the 1β-hydroxy-deoxycholic acid (1β-OH-DCA) to deoxycholic acid (DCA) urinary metabolic ratio (UMR) as a CYP3A metric in ten male healthy volunteers. Midazolam (MDZ) 1 mg was administered orally at three sessions: alone (control session), after pre-treatment with fluvoxamine 50 mg (12 h and 2 h prior to MDZ administration), and voriconazole 400 mg (2 h before MDZ administration) (inhibition session), and after a 7-day pre-treatment with the inducer rifampicin 600 mg (induction session). The 1β-OH-DCA/DCA UMR was measured at each session, and correlations with MDZ metrics were established. At baseline, the 1β-OH-DCA/DCA UMR correlated significantly with oral MDZ clearance (r = 0.652, p = 0.041) and Cmax (r = -0.652, p = 0.041). In addition, the modulation of CYP3A was reflected in the 1β-OH-DCA/DCA UMR after the intake of rifampicin (induction ratio = 11.4, p < 0.01). During the inhibition session, a non-significant 22% decrease in 1β-OH-DCA/DCA was observed (p = 0.275). This result could be explained by the short duration of CYP3A inhibitors intake fixed in our clinical trial. Additional studies, particularly involving CYP3A inhibition for a longer period and larger sample sizes, are needed to confirm the 1β-OH-DCA/DCA metric as a suitable CYP3A biomarker.
Keywords: CYP3A; CYP450; bile acid; biomarker; phenotyping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

