Flavan-3-ols and Proanthocyanidins in Japanese, Bohemian and Giant Knotweed
- PMID: 33672472
- PMCID: PMC7923414
- DOI: 10.3390/plants10020402
Flavan-3-ols and Proanthocyanidins in Japanese, Bohemian and Giant Knotweed
Abstract
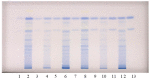
Flavan-3-ols and proanthocyanidins of invasive alien plants Japanese knotweed (Fallopia japonica Houtt.), giant knotweed (Fallopia sachalinensis F. Schmidt) and Bohemian knotweed (Fallopia × bohemica (Chrtek & Chrtkova) J.P. Bailey) were investigated using high performance thin-layer chromatography (HPTLC) coupled to densitometry, image analysis and mass spectrometry (HPTLC-MS/MS). (+)-Catechin, (-)-epicatechin, (-)-epicatechin gallate and procyanidin B2 were found in rhizomes of these three species, and for the first time in Bohemian knotweed. (-)-Epicatechin gallate, procyanidin B1, procyanidin B2 and procyanidin C1 were found in giant knotweed rhizomes for the first time. Rhizomes of Bohemian and giant knotweed have the same chemical profiles of proanthocyanidins with respect to the degree of polymerization and with respect to gallates. Japanese and Bohemian knotweed have equal chromatographic fingerprint profiles with the additional peak not present in giant knotweed. Within the individual species giant knotweed rhizomes and leaves have the most similar fingerprints, while the fingerprints of Japanese and Bohemian knotweed rhizomes have additional peaks not found in leaves. Rhizomes of all three species proved to be a rich source of proanthocyanidins, with the highest content in Japanese and the lowest in Bohemian knotweed (based on the total peak areas). The contents of monomers in Japanese, Bohemian and giant knotweed rhizomes were 2.99 kg/t of dry mass (DM), 1.52 kg/t DM, 2.36 kg/t DM, respectively, while the contents of dimers were 2.81 kg/t DM, 1.09 kg/t DM, 2.17 kg/t DM, respectively. All B-type proanthocyanidins from monomers to decamers (monomers-flavan-3-ols, dimers, trimers, tetramers, pentamers, hexamers, heptamers, octamers, nonamers and decamers) and some of their gallates (monomer gallates, dimer gallates, dimer digallates, trimer gallates, tetramer gallates, pentamer gallates and hexamer gallates) were identified in rhizomes of Bohemian knotweed and giant knotweed. Pentamer gallates, hexamers, hexamer gallates, nonamers and decamers were identified for the first time in this study in Bohemian and giant knotweed rhizomes.
Keywords: HPTLC–MS; Polygonaceae; Polygonum; Reynoutria; catechins; chemical profiling; condensed tannins; fingerprints; flavanols; procyanidins.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








References
-
- Balogh L. Japanese, giant and Bohemian knotweed (Fallopia japonica (Houtt.) Ronse Decr., F. sachalinensis (Frdr. Schmidt) Ronse Decr. and F. × bohemica (Chrtek et Chrtková) J. P. Bailey) In: Botta-Dukát Z., Balogh L., editors. The Most Invasive Plants in Hungary. 1st ed. Institute of Ecology and Botany, Hungarian Academy of Sciences; Vácrátót, Hungary: 2008. pp. 13–33.
-
- Global Invasive Species Database. [(accessed on 12 January 2021)]; Available online: http://www.iucngisd.org/gisd/search.php.
-
- Alberternst B., Böhmer H.J. NOBANIS—Invasive Alien Species Fact Sheet—Fallopia japonica. [(accessed on 12 January 2021)]; Available online: https://www.nobanis.org/globalassets/speciesinfo/r/reynoutria-japonica/r....
-
- Shaw R.H., Bryner S., Tanner R. The life history and host range of the Japanese knotweed psyllid, Aphalara itadori Shinji: Potentially the first classical biological weed control agent for the European Union. Biol. Control. 2009;49:105–113. doi: 10.1016/j.biocontrol.2009.01.016. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

