Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies
- PMID: 33672611
- PMCID: PMC7924201
- DOI: 10.3390/ijms22042096
Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies
Abstract
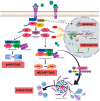
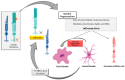
Inherited retinal dystrophies (IRDs) are a group of retinal disorders that cause progressive and severe loss of vision because of retinal cell death, mainly photoreceptor cells. IRDs include retinitis pigmentosa (RP), the most common IRD. IRDs present a genetic and clinical heterogeneity that makes it difficult to achieve proper treatment. The progression of IRDs is influenced, among other factors, by the activation of the immune cells (microglia, macrophages, etc.) and the release of inflammatory molecules such as chemokines and cytokines. Upregulation of tumor necrosis factor alpha (TNFα), a pro-inflammatory cytokine, is found in IRDs. This cytokine may influence photoreceptor cell death. Different cell death mechanisms are proposed, including apoptosis, necroptosis, pyroptosis, autophagy, excessive activation of calpains, or parthanatos for photoreceptor cell death. Some of these cell death mechanisms are linked to TNFα upregulation and inflammation. Therapeutic approaches that reduce retinal inflammation have emerged as useful therapies for slowing down the progression of IRDs. We focused this review on the relationship between retinal inflammation and the different cell death mechanisms involved in RP. We also reviewed the main anti-inflammatory therapies for the treatment of IRDs.
Keywords: TNFα; cell death; inflammation; retinal dystrophies.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- RetNet Retinal Information Network. [(accessed on 29 December 2020)]; Available online: https://sph.uth.edu/retnet/home.htm.
-
- Chang B. Animal Models of Retinitis Pigmentosa (RP) In: Chan C.-C., editor. Animal Models of Ophthalmic Diseases. Springer International Publishing; Cham, Switzerland: 2016. pp. 101–116.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

