Neuroimmune Regulation of Surgery-Associated Metastases
- PMID: 33672617
- PMCID: PMC7924204
- DOI: 10.3390/cells10020454
Neuroimmune Regulation of Surgery-Associated Metastases
Abstract
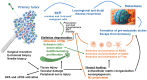
Surgery remains an essential therapeutic approach for most solid malignancies. Although for more than a century accumulating clinical and experimental data have indicated that surgical procedures themselves may promote the appearance and progression of recurrent and metastatic lesions, only in recent years has renewed interest been taken in the mechanism by which metastasizing of cancer occurs following operative procedures. It is well proven now that surgery constitutes a risk factor for the promotion of pre-existing, possibly dormant micrometastases and the acceleration of new metastases through several mechanisms, including the release of neuroendocrine and stress hormones and wound healing pathway-associated immunosuppression, neovascularization, and tissue remodeling. These postoperative consequences synergistically facilitate the establishment of new metastases and the development of pre-existing micrometastases. While only in recent years the role of the peripheral nervous system has been recognized as another contributor to cancer development and metastasis, little is known about the contribution of tumor-associated neuronal and neuroglial elements in the metastatic disease related to surgical trauma and wound healing. Specifically, although numerous clinical and experimental data suggest that biopsy- and surgery-induced wound healing can promote survival and metastatic spread of residual and dormant malignant cells, the involvement of the tumor-associated neuroglial cells in the formation of metastases following tissue injury has not been well understood. Understanding the clinical significance and underlying mechanisms of neuroimmune regulation of surgery-associated metastasis will not only advance the field of neuro-immuno-oncology and contribute to basic science and translational oncology research but will also produce a strong foundation for developing novel mechanism-based therapeutic approaches that may protect patients against the oncologically adverse effects of primary tumor biopsy and excision.
Keywords: metastasis; neoneurogenesis; neuroglia; neuroimmune axis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Modeling growth kinetics and statistical distribution of oligometastases.Semin Radiat Oncol. 2006 Apr;16(2):111-9. doi: 10.1016/j.semradonc.2005.12.006. Semin Radiat Oncol. 2006. PMID: 16564446 Review.
-
Surgery, wound healing, and metastasis: recent insights and clinical implications.Crit Rev Oncol Hematol. 2014 Jan;89(1):16-26. doi: 10.1016/j.critrevonc.2013.07.008. Epub 2013 Aug 16. Crit Rev Oncol Hematol. 2014. PMID: 23958676 Review.
-
Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement.Brain Behav Immun. 2013 Mar;30 Suppl(Suppl):S32-40. doi: 10.1016/j.bbi.2012.03.006. Epub 2012 Apr 4. Brain Behav Immun. 2013. PMID: 22504092 Free PMC article. Review.
-
Role of myeloid-derived suppressor cells in metastasis.Cancer Metastasis Rev. 2021 Jun;40(2):391-411. doi: 10.1007/s10555-020-09947-x. Epub 2021 Jan 7. Cancer Metastasis Rev. 2021. PMID: 33411082 Review.
-
The Neuroimmune Axis in the Tumor Microenvironment.J Immunol. 2020 Jan 15;204(2):280-285. doi: 10.4049/jimmunol.1900828. J Immunol. 2020. PMID: 31907270 Review.
Cited by
-
Schwann cells in the normal and pathological lung microenvironment.Front Mol Biosci. 2024 Apr 4;11:1365760. doi: 10.3389/fmolb.2024.1365760. eCollection 2024. Front Mol Biosci. 2024. PMID: 38638689 Free PMC article. Review.
-
Schwann Cells in the Tumor Microenvironment: Need More Attention.J Oncol. 2022 Feb 10;2022:1058667. doi: 10.1155/2022/1058667. eCollection 2022. J Oncol. 2022. PMID: 35186076 Free PMC article. Review.
-
Neurogenesis manifestations of solid tumor and tracer imaging studies: a narrative review.Am J Cancer Res. 2023 Mar 15;13(3):713-726. eCollection 2023. Am J Cancer Res. 2023. PMID: 37034231 Free PMC article. Review.
-
Colorectal cancer and dormant metastases: Put to sleep or destroy?World J Gastrointest Oncol. 2024 Jun 15;16(6):2304-2317. doi: 10.4251/wjgo.v16.i6.2304. World J Gastrointest Oncol. 2024. PMID: 38994146 Free PMC article.
-
Liver Metastasis in Cancer: Molecular Mechanisms and Management.MedComm (2020). 2025 Feb 27;6(3):e70119. doi: 10.1002/mco2.70119. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40027151 Free PMC article. Review.
References
-
- American Cancer Society Official Page Cancer Facts and Figures 2020. [(accessed on 1 November 2020)]; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-....
-
- Simpson-Herren L., Sanford A.H., Holmquist J.P. Effects of surgery on the cell kinetics of residual tumor. Cancer Treat. Rep. 1976;60:1749–1760. - PubMed
-
- Horg S.A., Rubin P., DeWys W.D. Clinical Oncology for Medical Students and Physicians-A Multidisciplinary Approach. 6th ed. American Cancer Society; New York, NY, USA: 1983. Metastasis and disseminated disease; pp. 498–499.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

