CircAgtpbp1 Acts as a Molecular Sponge of miR-543-5p to Regulate the Secretion of GH in Rat Pituitary Cells
- PMID: 33672649
- PMCID: PMC7924184
- DOI: 10.3390/ani11020558
CircAgtpbp1 Acts as a Molecular Sponge of miR-543-5p to Regulate the Secretion of GH in Rat Pituitary Cells
Abstract
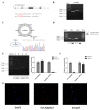
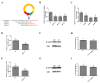
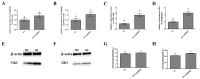
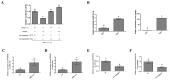
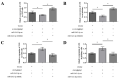
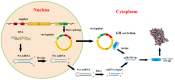
CircRNAs have been identified to be expressed differently and stably in numerous species and tissues, but their functions in growth hormone (GH) secretion are still largely unknown. In summary, we have revealed a circRNA-miRNA-mRNA network that may play a biological role in the rat pituitary gland. First, we verified the chromosome location information of circAgtpbp1 according to sequencing analysis. The circAgtpbp1 characteristics were authenticated through PCR, qRT-PCR, treating with RNase and fluorescent in situ hybridization (FISH). Second, we detected the expression pattern of circAgtpbp1 in the rat anterior pituitary by qRT-PCR. We also designed circAgtpbp1 siRNA and constructed overexpression plasmid to evaluate the effect of circAgtpbp1 function on GH secretion by qRT-PCR, ELISA and Western blot. CircAgtpbp1 is a stable, truly circular molecule. We found that circAgtpbp1 interacted with miR-543-5p and can regulate GH secretion in pituitary cells through a circAgtpbp1-miR-543-5p-GH axis. Overall, the evidence generated by our study suggests that circAgtpbp1 can act as a sponge of miR-543-5p to reduce the inhibitory effect of miR-543-5p on Gh1 and further promote GH secretion. These findings expand our existing knowledge on the mechanisms of hormone regulation in the pituitary gland.
Keywords: GH; animal growth; circRNA; miRNA sponge; pituitary.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Roles of differential expression of miR-543-5p in GH regulation in rat anterior pituitary cells and GH3 cells.PLoS One. 2019 Sep 11;14(9):e0222340. doi: 10.1371/journal.pone.0222340. eCollection 2019. PLoS One. 2019. PMID: 31509580 Free PMC article.
-
circAGTPBP1 promotes the progression of papillary thyroid cancer through the notch pathway via the miR-34a-5p/notch1 axis.iScience. 2023 Aug 9;26(9):107564. doi: 10.1016/j.isci.2023.107564. eCollection 2023 Sep 15. iScience. 2023. PMID: 37622004 Free PMC article.
-
circAkap17b acts as a miR-7 family molecular sponge to regulate FSH secretion in rat pituitary cells.J Mol Endocrinol. 2020 Nov;65(4):135-148. doi: 10.1530/JME-20-0036. J Mol Endocrinol. 2020. PMID: 33048061
-
Alteration of the miRNA expression profile in male porcine anterior pituitary cells in response to GHRH and CST and analysis of the potential roles for miRNAs in regulating GH.Growth Horm IGF Res. 2015 Apr;25(2):66-74. doi: 10.1016/j.ghir.2014.12.002. Epub 2014 Dec 9. Growth Horm IGF Res. 2015. PMID: 25613666
-
Circular RNA BCRC-3 suppresses bladder cancer proliferation through miR-182-5p/p27 axis.Mol Cancer. 2018 Oct 3;17(1):144. doi: 10.1186/s12943-018-0892-z. Mol Cancer. 2018. PMID: 30285878 Free PMC article.
Cited by
-
Rno_circ_0001004 Acts as a miR-709 Molecular Sponge to Regulate the Growth Hormone Synthesis and Cell Proliferation.Int J Mol Sci. 2022 Jan 26;23(3):1413. doi: 10.3390/ijms23031413. Int J Mol Sci. 2022. PMID: 35163336 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

