Lysophosphatidic Acid-Activated Calcium Signaling Is Elevated in Red Cells from Sickle Cell Disease Patients
- PMID: 33672679
- PMCID: PMC7924404
- DOI: 10.3390/cells10020456
Lysophosphatidic Acid-Activated Calcium Signaling Is Elevated in Red Cells from Sickle Cell Disease Patients
Abstract
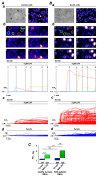
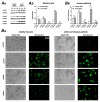
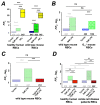
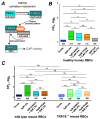
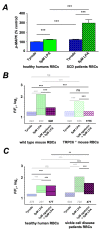
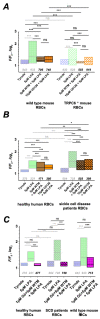
(1) Background: It is known that sickle cells contain a higher amount of Ca2+ compared to healthy red blood cells (RBCs). The increased Ca2+ is associated with the most severe symptom of sickle cell disease (SCD), the vaso-occlusive crisis (VOC). The Ca2+ entry pathway received the name of Psickle but its molecular identity remains only partly resolved. We aimed to map the involved Ca2+ signaling to provide putative pharmacological targets for treatment. (2) Methods: The main technique applied was Ca2+ imaging of RBCs from healthy donors, SCD patients and a number of transgenic mouse models in comparison to wild-type mice. Life-cell Ca2+ imaging was applied to monitor responses to pharmacological targeting of the elements of signaling cascades. Infection as a trigger of VOC was imitated by stimulation of RBCs with lysophosphatidic acid (LPA). These measurements were complemented with biochemical assays. (3) Results: Ca2+ entry into SCD RBCs in response to LPA stimulation exceeded that of healthy donors. LPA receptor 4 levels were increased in SCD RBCs. Their activation was followed by the activation of Gi protein, which in turn triggered opening of TRPC6 and CaV2.1 channels via a protein kinase Cα and a MAP kinase pathway, respectively. (4) Conclusions: We found a new Ca2+ signaling cascade that is increased in SCD patients and identified new pharmacological targets that might be promising in addressing the most severe symptom of SCD, the VOC.
Keywords: CaV2.1; G protein signaling; Gárdos channel; LPA receptor; MAP kinase; TRPC6; erythrocytes; protein kinase Cα; sickle cell disease; transgenic mice.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

