Advances and Perspectives in the Management of Varicella-Zoster Virus Infections
- PMID: 33672709
- PMCID: PMC7924330
- DOI: 10.3390/molecules26041132
Advances and Perspectives in the Management of Varicella-Zoster Virus Infections
Abstract
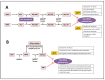
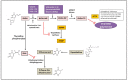
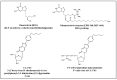
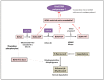
Varicella-zoster virus (VZV), a common and ubiquitous human-restricted pathogen, causes a primary infection (varicella or chickenpox) followed by establishment of latency in sensory ganglia. The virus can reactivate, causing herpes zoster (HZ, shingles) and leading to significant morbidity but rarely mortality, although in immunocompromised hosts, VZV can cause severe disseminated and occasionally fatal disease. We discuss VZV diseases and the decrease in their incidence due to the introduction of live-attenuated vaccines to prevent varicella or HZ. We also focus on acyclovir, valacyclovir, and famciclovir (FDA approved drugs to treat VZV infections), brivudine (used in some European countries) and amenamevir (a helicase-primase inhibitor, approved in Japan) that augur the beginning of a new era of anti-VZV therapy. Valnivudine hydrochloride (FV-100) and valomaciclovir stearate (in advanced stage of development) and several new molecules potentially good as anti-VZV candidates described during the last year are examined. We reflect on the role of antiviral agents in the treatment of VZV-associated diseases, as a large percentage of the at-risk population is not immunized, and on the limitations of currently FDA-approved anti-VZV drugs. Their low efficacy in controlling HZ pain and post-herpetic neuralgia development, and the need of multiple dosing regimens requiring daily dose adaptation for patients with renal failure urges the development of novel anti-VZV drugs.
Keywords: HZ; amenamevir; anti-VZV drugs; chickenpox; helicase-primase inhibitors; nucleoside analogues; shingles; valnivudine hydrochloride (FV-100); valomaciclovir stearate; varicella-zoster virus (VZV).
Conflict of interest statement
The authors declare no conflict of interest
Figures
References
-
- Breuer J., Whitley R. Varicella zoster virus: Natural history and current therapies of varicella and herpes zoster. Herpes. 2007;14:25–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

