A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology
- PMID: 33672726
- PMCID: PMC7924394
- DOI: 10.3390/molecules26041139
A Novel Thermostable and Alkaline Protease Produced from Bacillus stearothermophilus Isolated from Olive Oil Mill Sols Suitable to Industrial Biotechnology
Abstract
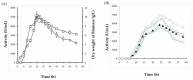
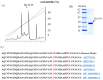
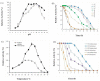
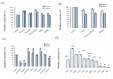
This study was conducted to identify a new alkaline and thermophilic protease (Ba.St.Pr) produced from Bacillus stearothermophilus isolated from olive oil mill sols and to evaluate its culture conditions, including temperature, pH, carbon and nitrogen sources, and incubation time. The optimum culture conditions for cell growth (10 g/L) and protease production (5050 U/mL) were as follows: temperature 55 °C, pH 10, inoculation density 8 × 108 CFU/mL, and incubation time 24 h. The use of 3% yeast extract as the nitrogen sources and galactose (7.5 g/L) as the carbon sources enhanced both cell growth and protease production. Using reversed-phase analytical HPLC on C-8 column, the new protease was purified with a molecular mass of approximately 28 kDa. The N-terminal sequence of Ba.St.Pr exhibited a high level of identity of approximately 95% with those of Bacillus strains. Characterization under extreme conditions revealed a novel thermostable and alkaline protease with a half-life time of 187 min when incubated with combined Ca2+/mannitol. Ba.St.Pr demonstrated a higher stability in the presence of surfactant, solvent, and Ca2+ ions. Consequently, all the evaluated activity parameters highlighted the promising properties of this bacterium for industrial and biotechnological applications.
Keywords: Bacillus stearothermophilus; characterization; protease; thermostable.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tufvesson P., Lima-Ramos J., Nordblad M., Woodley J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Process. Res. Dev. 2011;15:266–274. doi: 10.1021/op1002165. - DOI
-
- Belmessikh A.B., Boukhalfa H., Mechakra-Maza A., Gheribi-Aoulmi Z., Amrane A. statistical optimization of culture medium for neutral protease production by Aspergillus oryzae. Comparative study between solid and submerged fermentations on tomato pomace. J. Taiwan Inst. Chem. Eng. 2013;44:377–385. doi: 10.1016/j.jtice.2012.12.011. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

