Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study
- PMID: 33672783
- PMCID: PMC7924657
- DOI: 10.3390/molecules26041144
Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study
Abstract
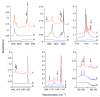
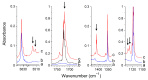
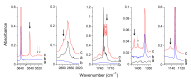
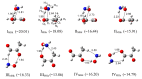
The interactions of formaldehyde (FA), glyoxal (Gly) and methylglyoxal (MGly) with hydroxylamine (HA) isolated in solid argon and nitrogen were studied using FTIR spectroscopy and ab initio methods. The spectra analysis indicates the formation of two types of hydrogen-bonded complexes between carbonyl and hydroxylamine in the studied matrices. The cyclic planar complexes are stabilized by O-H⋯O(C), and C-H⋯N interactions and the nonplanar complexes are stabilized by O-H⋯O(C) bond. Formaldehyde was found to form with hydroxylamine, the cyclic planar complex and methylglyoxal, the nonplanar one in both argon and nitrogen matrices. In turn, glyoxal forms with hydroxylamine the most stable nonplanar complex in solid argon, whereas in solid nitrogen, both types of the complex are formed.
Keywords: carbonyls; computational chemistry; hydrogen bond; hydroxylamine; matrix isolation; vibrational spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Photo-Induced Reactions between Glyoxal and Hydroxylamine in Cryogenic Matrices.Molecules. 2022 Jul 27;27(15):4797. doi: 10.3390/molecules27154797. Molecules. 2022. PMID: 35956748 Free PMC article.
-
Complexes of atmospheric alpha-dicarbonyls with water: FTIR matrix isolation and theoretical study.J Phys Chem A. 2007 Mar 29;111(12):2398-406. doi: 10.1021/jp066685s. Epub 2007 Mar 3. J Phys Chem A. 2007. PMID: 17388308
-
Experimental FTIR-MI and Theoretical Studies of Isocyanic Acid Aggregates.Molecules. 2023 Feb 2;28(3):1430. doi: 10.3390/molecules28031430. Molecules. 2023. PMID: 36771094 Free PMC article.
-
Structure and IR Spectroscopic Properties of HNCO Complexes with SO2 Isolated in Solid Argon.Molecules. 2021 Oct 25;26(21):6441. doi: 10.3390/molecules26216441. Molecules. 2021. PMID: 34770850 Free PMC article.
-
Structural and spectroscopic characterization of DMF complexes with nitrogen, carbon monoxide, ammonia and water. Infrared matrix isolation and theoretical studies [corrected].Spectrochim Acta A Mol Biomol Spectrosc. 2018 Feb 5;190:423-432. doi: 10.1016/j.saa.2017.09.046. Epub 2017 Sep 18. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 28957704
Cited by
-
Photo-Induced Reactions between Glyoxal and Hydroxylamine in Cryogenic Matrices.Molecules. 2022 Jul 27;27(15):4797. doi: 10.3390/molecules27154797. Molecules. 2022. PMID: 35956748 Free PMC article.
-
Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets.Polymers (Basel). 2025 Apr 11;17(8):1038. doi: 10.3390/polym17081038. Polymers (Basel). 2025. PMID: 40284302 Free PMC article.
References
-
- Whittle E., Dows D.A., Pimentel G.C. Matrix isolation method for the experimental study of unstable species. J. Chem. Phys. 1954;22:1943. doi: 10.1063/1.1739957. - DOI
-
- Ault B.S. Matrix isolation studies of reactive intermediate complexes. Rev. Chem. Intermed. 1988;9:233–269. doi: 10.1007/BF03155685. - DOI
-
- Barnes A.J. Matrix isolation studies of hydrogen bonding—An historical perspective. J. Mol. Struct. 2018;1163:77–85. doi: 10.1016/j.molstruc.2018.01.045. - DOI
-
- Rosenberg S., Silver S.M., Sayer J.M., Jencks W.P. Evidence for two concurrent mechanisms and a kinetically significant proton transfer process in acid-catalyzed o-methyloxime formation. J. Am. Chem. Soc. 1974;96:7986–7998. doi: 10.1021/ja00833a026. - DOI
-
- Sayer J.M., Pinsky B., Schonbrunn A., Washtien W. Mechanism of carbinolamine formation. J. Am. Chem. Soc. 1974;96:7998–8009. doi: 10.1021/ja00833a027. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

