Biocatalytic C-C Bond Formation for One Carbon Resource Utilization
- PMID: 33672882
- PMCID: PMC7918591
- DOI: 10.3390/ijms22041890
Biocatalytic C-C Bond Formation for One Carbon Resource Utilization
Abstract
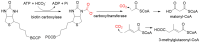
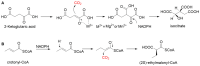
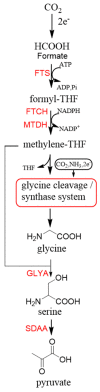
The carbon-carbon bond formation has always been one of the most important reactions in C1 resource utilization. Compared to traditional organic synthesis methods, biocatalytic C-C bond formation offers a green and potent alternative for C1 transformation. In recent years, with the development of synthetic biology, more and more carboxylases and C-C ligases have been mined and designed for the C1 transformation in vitro and C1 assimilation in vivo. This article presents an overview of C-C bond formation in biocatalytic C1 resource utilization is first provided. Sets of newly mined and designed carboxylases and ligases capable of catalyzing C-C bond formation for the transformation of CO2, formaldehyde, CO, and formate are then reviewed, and their catalytic mechanisms are discussed. Finally, the current advances and the future perspectives for the development of catalysts for C1 resource utilization are provided.
Keywords: C-C ligases; C1 resource utilization; carboxylases; designed pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- OECD . The Bioeconomy to 2030: Designing a Policy Agenda. OECD; Paris, France: 2009. pp. 1–322.
-
- Zhang W., Zhang T., Wu S., Wu M., Xin F., Dong W., Ma J., Zhang M., Jiang M. Guidance for engineering of synthetic methylotrophy based on methanol metabolism in methylotrophy. RSC Adv. 2017;7:4083–4091. doi: 10.1039/C6RA27038G. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

