Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer
- PMID: 33672918
- PMCID: PMC7917935
- DOI: 10.3390/polym13040572
Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer
Abstract
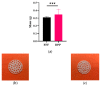
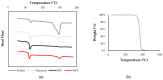
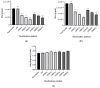
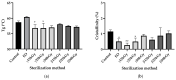
Although bioabsorbable polymers have garnered increasing attention because of their potential in tissue engineering applications, to our knowledge there are only a few bioabsorbable 3D printed medical devices on the market thus far. In this study, we assessed the processability of medical grade Poly(lactic-co-glycolic) Acid (PLGA)85:15 via two additive manufacturing technologies: Fused Filament Fabrication (FFF) and Direct Pellet Printing (DPP) to highlight the least destructive technology towards PLGA. To quantify PLGA degradation, its molecular weight (gel permeation chromatography (GPC)) as well as its thermal properties (differential scanning calorimetry (DSC)) were evaluated at each processing step, including sterilization with conventional methods (ethylene oxide, gamma, and beta irradiation). Results show that 3D printing of PLGA on a DPP printer significantly decreased the number-average molecular weight (Mn) to the greatest extent (26% Mn loss, p < 0.0001) as it applies a longer residence time and higher shear stress compared to classic FFF (19% Mn loss, p < 0.0001). Among all sterilization methods tested, ethylene oxide seems to be the most appropriate, as it leads to no significant changes in PLGA properties. After sterilization, all samples were considered to be non-toxic, as cell viability was above 70% compared to the control, indicating that this manufacturing route could be used for the development of bioabsorbable medical devices. Based on our observations, we recommend using FFF printing and ethylene oxide sterilization to produce PLGA medical devices.
Keywords: additive manufacturing; bioabsorbable; medical devices; polymer; sterilization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Franchetti M., Kress C. An Economic Analysis Comparing the Cost Feasibility of Replacing Injection Molding Processes with Emerging Additive Manufacturing Techniques. Int. J. Adv. Manuf. Technol. 2017;88:2573–2579. doi: 10.1007/s00170-016-8968-7. - DOI
-
- Nikoubashman O., Heringer S., Feher K., Brockmann M.-A., Sellhaus B., Dreser A., Kurtenbach K., Pjontek R., Jockenhövel S., Weis J., et al. Development of a Polymer-Based Biodegradable Neurovascular Stent Prototype: A Preliminary In Vitro and In Vivo Study. Macromol. Biosci. 2018;18:1700292. doi: 10.1002/mabi.201700292. - DOI - PubMed
-
- Liao L., Peng C., Li S., Lu Z., Fan Z. Evaluation of Bioresorbable Polymers as Potential Stent Material—In Vivo Degradation Behavior and Histocompatibility. J. Appl. Polym. Sci. 2017;134 doi: 10.1002/app.44355. - DOI
-
- Maurus P.B., Kaeding C.C. Bioabsorbable Implant Material Review. Oper. Tech. Sports Med. 2004;12:158–160. doi: 10.1053/j.otsm.2004.07.015. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

