Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis
- PMID: 33672962
- PMCID: PMC7918628
- DOI: 10.3390/ijms22041897
Transglutaminase 2 as a Marker for Inflammation and Therapeutic Target in Sepsis
Abstract
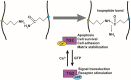
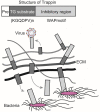
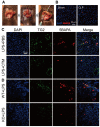
Sepsis results in lethal organ malfunction due to dysregulated host response to infection, which is a condition with increasing prevalence worldwide. Transglutaminase 2 (TG2) is a crosslinking enzyme that forms a covalent bond between lysine and glutamine. TG2 plays important roles in diverse cellular processes, including extracellular matrix stabilization, cytoskeletal function, cell motility, adhesion, signal transduction, apoptosis, and cell survival. We have shown that the co-culture of Candida albicans and hepatocytes activates and induces the translocation of TG2 into the nucleus. In addition, the expression and activation of TG2 in liver macrophages was dramatically induced in the lipopolysaccharide-injected and cecal ligation puncture-operated mouse models of sepsis. Based on these findings and recently published research, we have reviewed the current understanding of the relationship between TG2 and sepsis. Following the genetic and pharmacological inhibition of TG2, we also assessed the evidence regarding the use of TG2 as a potential marker and therapeutic target in inflammation and sepsis.
Keywords: Elafin; antibacterial; antiviral; covalent crosslinking; inhibitor; sepsis; transglutaminase.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures
Similar articles
-
Imaging of the ex vivo transglutaminase activity in liver macrophages of sepsis mice.Anal Biochem. 2020 May 15;597:113654. doi: 10.1016/j.ab.2020.113654. Epub 2020 Mar 3. Anal Biochem. 2020. PMID: 32142762
-
Transglutaminase 2 suppresses apoptosis by modulating caspase 3 and NF-kappaB activity in hypoxic tumor cells.Oncogene. 2010 Jan 21;29(3):356-67. doi: 10.1038/onc.2009.342. Epub 2009 Oct 19. Oncogene. 2010. PMID: 19838207
-
Transglutaminase Is Required for Epidermal Squamous Cell Carcinoma Stem Cell Survival.Mol Cancer Res. 2015 Jul;13(7):1083-94. doi: 10.1158/1541-7786.MCR-14-0685-T. Epub 2015 May 1. Mol Cancer Res. 2015. PMID: 25934691 Free PMC article.
-
Is monocyte- and macrophage-derived tissue transglutaminase involved in inflammatory processes?Amino Acids. 2017 Mar;49(3):441-452. doi: 10.1007/s00726-016-2334-9. Epub 2016 Sep 22. Amino Acids. 2017. PMID: 27659795 Free PMC article. Review.
-
Transglutaminase-2: evolution from pedestrian protein to a promising therapeutic target.Amino Acids. 2017 Mar;49(3):425-439. doi: 10.1007/s00726-016-2320-2. Epub 2016 Aug 25. Amino Acids. 2017. PMID: 27562794 Review.
Cited by
-
Inhibiting Transglutaminase 2 Mediates Kidney Fibrosis via Anti-Apoptosis.Biomedicines. 2022 Jun 7;10(6):1345. doi: 10.3390/biomedicines10061345. Biomedicines. 2022. PMID: 35740367 Free PMC article.
-
The upregulation of TGM2 is associated with poor prognosis and the shaping of the inflammatory tumor microenvironment in lung squamous cell carcinoma.Am J Cancer Res. 2024 Jun 15;14(6):2823-2838. doi: 10.62347/OBES4130. eCollection 2024. Am J Cancer Res. 2024. PMID: 39005693 Free PMC article.
-
Immuno-Sensing at Ultra-Low Concentration of TG2 Protein by Organic Electrochemical Transistors.Biosensors (Basel). 2023 Mar 31;13(4):448. doi: 10.3390/bios13040448. Biosensors (Basel). 2023. PMID: 37185523 Free PMC article.
-
Necrotizing enterocolitis: diagnosis with plasma IgG anti-tissue transglutaminase antibodies.Pediatr Res. 2025 Apr 15. doi: 10.1038/s41390-025-04040-x. Online ahead of print. Pediatr Res. 2025. PMID: 40234717
-
Transglutaminase 2-expressing macrophages modulate adipose tissue inflammation.Commun Biol. 2025 Jun 4;8(1):859. doi: 10.1038/s42003-025-08199-1. Commun Biol. 2025. PMID: 40467990 Free PMC article.
References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

