Bi-Functional Radiotheranostics of 188Re-Liposome-Fcy-hEGF for Radio- and Chemo-Therapy of EGFR-Overexpressing Cancer Cells
- PMID: 33672989
- PMCID: PMC7918434
- DOI: 10.3390/ijms22041902
Bi-Functional Radiotheranostics of 188Re-Liposome-Fcy-hEGF for Radio- and Chemo-Therapy of EGFR-Overexpressing Cancer Cells
Abstract
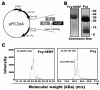
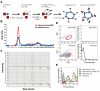
Epidermal growth factor receptor (EGFR) specific therapeutics is of great importance in cancer treatment. Fcy-hEGF fusion protein, composed of yeast cytosine deaminase (Fcy) and human EGF (hEGF), is capable of binding to EGFR and enzymatically convert 5-fluorocytosine (5-FC) to 1000-fold toxic 5-fluorocuracil (5-FU), thereby inhibiting the growth of EGFR-expressing tumor cells. To develop EGFR-specific therapy, 188Re-liposome-Fcy-hEGF was constructed by insertion of Fcy-hEGF fusion protein onto the surface of liposomes encapsulating of 188Re. Western blotting, MALDI-TOF, column size exclusion and flow cytometry were used to confirm the conjugation and bio-activity of 188Re-liposome-Fcy-hEGF. Cell lines with EGFR expression were subjected to treat with 188Re-liposome-Fcy-hEGF/5-FC in the presence of 5-FC. The 188Re-liposome-Fcy-hEGF/5-FC revealed a better cytotoxic effect for cancer cells than the treatment of liposome-Fcy-hEGF/5-FC or 188Re-liposome-Fcy-hEGF alone. The therapeutics has radio- and chemo-toxicity simultaneously and specifically target to EGFR-expression tumor cells, thereby achieving synergistic anticancer activity.
Keywords: 5-fluorocuracil; cytosine deaminase; epidermal growth factor receptor; liposome; prodrug; rhenium-188.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

