Resveratrol Inhibits Venezuelan Equine Encephalitis Virus Infection by Interfering with the AKT/GSK Pathway
- PMID: 33673026
- PMCID: PMC7918260
- DOI: 10.3390/plants10020346
Resveratrol Inhibits Venezuelan Equine Encephalitis Virus Infection by Interfering with the AKT/GSK Pathway
Abstract
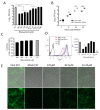
The host proteins Protein Kinase B (AKT) and glycogen synthase kinase-3 (GSK-3) are associated with multiple neurodegenerative disorders. They are also important for the replication of Venezuelan equine encephalitis virus (VEEV), thereby making the AKT/GSK-3 pathway an attractive target for developing anti-VEEV therapeutics. Resveratrol, a natural phytochemical, has been shown to substantially inhibit the AKT pathway. Therefore, we attempted to explore whether it exerts any antiviral activity against VEEV. In this study, we utilized green fluorescent protein (GFP)- and luciferase-encoding recombinant VEEV to determine the cytotoxicity and antiviral efficacy via luciferase reporter assays, flow cytometry, and immunofluorescent assays. Our results indicate that resveratrol treatment is capable of inhibiting VEEV replication, resulting in increased viability of Vero and U87MG cells as well as reduced virion production and viral RNA contents within host cells for at least 48 h with a single treatment. Furthermore, the suppression of apoptotic signaling adaptors, caspase-3, caspase-7, and annexin V may also be implicated in resveratrol-mediated antiviral activity. We found that decreased phosphorylation of the AKT/GSK-3 pathway, mediated by resveratrol, can be triggered during the early stages of VEEV infection, suggesting that resveratrol disrupts the viral replication cycle and consequently promotes cell survival. Finally, molecular docking and dynamics simulation studies revealed that resveratrol can directly bind to VEEV glycoproteins, which may interfere with virus attachment and entry. In conclusion, our results suggest that resveratrol exerts inhibitory activity against VEEV infection and upon further modification could be a useful compound to study in neuroprotective research and veterinary sciences.
Keywords: Akt; Venezuelan equine encephalitis virus; antiviral; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Estrada-Franco J.G., Navarro-Lopez R., Freier J.E., Cordova D., Clements T., Moncayo A., Kang W., Gomez-Hernandez C., Rodriguez-Dominguez G., Ludwig G.V., et al. Venezuelan equine encephalitis virus, southern Mexico. Emerg. Infect. Dis. 2004;10:2113–2121. doi: 10.3201/eid1012.040393. - DOI - PMC - PubMed
-
- Weaver S.C. Emerging Infections 1. ASM Press; Washington, DC, USA: 1998. Recurrent Emergence of Venezuelan Equine Encephalomyelitis; pp. 27–42. - DOI
-
- Johnson K.M., Martin D.H. Venezuelan equine encephalitis. Adv. Vet. Sci. Comp. Med. 1974;18:79–116. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

