SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond
- PMID: 33673048
- PMCID: PMC7918810
- DOI: 10.3390/vaccines9020147
SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond
Abstract
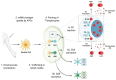
To successfully protect against pathogen infection, a vaccine must elicit efficient adaptive immunity, including B and T cell responses. While B cell responses are key, as they can mediate antibody-dependent protection, T cells can modulate B cell activity and directly contribute to the elimination of pathogen-infected cells. In the unprecedented race to develop an effective vaccine for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the respiratory disease coronavirus disease 2019 (COVID-19), messenger RNA (mRNA) vaccines have emerged as front runners thanks to their capacity for rapid development and ability to drive potent adaptive immune responses. In this review article, we provide an overview of the results from pre-clinical studies in animal models as well as clinical studies in humans that assessed the efficacy of SARS-CoV-2 mRNA vaccines, with a primary focus on adaptive immune responses post vaccination.
Keywords: SARS-CoV-2; T follicular helper cells; Th1 cells; adaptive immunity; antibodies; coronavirus; germinal centers; long-lived plasma cells; mRNA vaccines; memory B cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [(accessed on 31 December 2020)]; Available online: https://Coronavirus.Jhu.Edu/Map.Html.
-
- Draft Landscape of COVID-19 Candidate Vaccines. [(accessed on 29 December 2020)]; Available online: https://www.Who.Int/Publications/m/Item/Draft-Landscape-of-Covid-19-Cand....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

