Phenotypic Plasticity of Cancer Cells Based on Remodeling of the Actin Cytoskeleton and Adhesive Structures
- PMID: 33673054
- PMCID: PMC7918886
- DOI: 10.3390/ijms22041821
Phenotypic Plasticity of Cancer Cells Based on Remodeling of the Actin Cytoskeleton and Adhesive Structures
Abstract
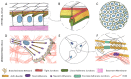
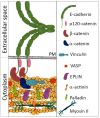
There is ample evidence that, instead of a binary switch, epithelial-mesenchymal transition (EMT) in cancer results in a flexible array of phenotypes, each one uniquely suited to a stage in the invasion-metastasis cascade. The phenotypic plasticity of epithelium-derived cancer cells gives them an edge in surviving and thriving in alien environments. This review describes in detail the actin cytoskeleton and E-cadherin-based adherens junction rearrangements that cancer cells need to implement in order to achieve the advantageous epithelial/mesenchymal phenotype and plasticity of migratory phenotypes that can arise from partial EMT.
Keywords: E-cadherin; EMT; actin cytoskeleton; adherens junctions; cancer cells; migration; plasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An In Vitro System to Study the Epithelial-Mesenchymal Transition In Vitro.Methods Mol Biol. 2018;1749:29-42. doi: 10.1007/978-1-4939-7701-7_4. Methods Mol Biol. 2018. PMID: 29525988
-
Cadherin-mediated cell-cell interactions in normal and cancer cells.Tissue Barriers. 2017 Jul 3;5(3):e1356900. doi: 10.1080/21688370.2017.1356900. Epub 2017 Jul 20. Tissue Barriers. 2017. PMID: 28783415 Free PMC article. Review.
-
Role of Epithelial-Mesenchymal Transition in Tumor Progression.Biochemistry (Mosc). 2018 Dec;83(12):1469-1476. doi: 10.1134/S0006297918120052. Biochemistry (Mosc). 2018. PMID: 30878022 Review.
-
Actin isoforms and reorganization of adhesion junctions in epithelial-to-mesenchymal transition of cervical carcinoma cells.Biochemistry (Mosc). 2012 Nov;77(11):1266-76. doi: 10.1134/S0006297912110053. Biochemistry (Mosc). 2012. PMID: 23240564
-
Dual role of E-cadherin in cancer cells.Tissue Barriers. 2022 Oct 2;10(4):2005420. doi: 10.1080/21688370.2021.2005420. Epub 2021 Nov 25. Tissue Barriers. 2022. PMID: 34821540 Free PMC article. Review.
Cited by
-
How does plasticity of migration help tumor cells to avoid treatment: Cytoskeletal regulators and potential markers.Front Pharmacol. 2022 Oct 6;13:962652. doi: 10.3389/fphar.2022.962652. eCollection 2022. Front Pharmacol. 2022. PMID: 36278174 Free PMC article. Review.
-
Molecular neuropathology of brain-invasive meningiomas.Brain Pathol. 2022 Mar;32(2):e13048. doi: 10.1111/bpa.13048. Brain Pathol. 2022. PMID: 35213084 Free PMC article. Review.
-
Unraveling the Role of the microRNA-Mediated Regulation of Actin-Binding Proteins in Ovarian Cancer: A Narrative Review.Cancers (Basel). 2025 Jul 11;17(14):2315. doi: 10.3390/cancers17142315. Cancers (Basel). 2025. PMID: 40723199 Free PMC article. Review.
-
ERK Signaling Pathway Is Constitutively Active in NT2D1 Non-Seminoma Cells and Its Inhibition Impairs Basal and HGF-Activated Cell Proliferation.Biomedicines. 2023 Jul 4;11(7):1894. doi: 10.3390/biomedicines11071894. Biomedicines. 2023. PMID: 37509533 Free PMC article.
-
Proteomics identifies apoptotic markers as predictors of histological transformation in patients with follicular lymphoma.Blood Adv. 2023 Dec 26;7(24):7418-7432. doi: 10.1182/bloodadvances.2023011299. Blood Adv. 2023. PMID: 37824846 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

